Cardiac safety assessment is a critical component of the drug development process, aimed at identifying potential adverse effects of pharmaceutical compounds on the heart. The significance of these evaluations lies in their capacity to prevent drug-induced cardiac arrhythmias, which can lead to serious health outcomes, including sudden cardiac death. By systematically identifying and mitigating these risks early in drug development, cardiac safety assessments protect patient health and contribute to the development of safer therapeutic options.
The landscape of cardiac safety assessment is critically informed by the ICH S7B guideline, an essential framework established to guide the non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by pharmaceuticals. This prolongation can increase the risk of cardiac arrhythmia, notably Torsades de Pointes (TdP), a potentially life-threatening condition. As part of a comprehensive safety evaluation, S7B offers a foundational approach for in vitro and in vivo studies to assess the risk of drugs to cause such adverse effects.
In 2022, the regulatory community welcomed the much-anticipated update to the S7B guideline, accompanied by a detailed Q&A document. This update represents a significant advancement in the field, incorporating the latest scientific understanding and methodologies. The 2022 S7B Q&A clarifies best practices and provides additional guidance on the implementation of these non-clinical evaluations. Key highlights include the emphasis on integrated risk assessments, leveraging both in vitro and in vivo data to predict QT interval prolongation more accurately.
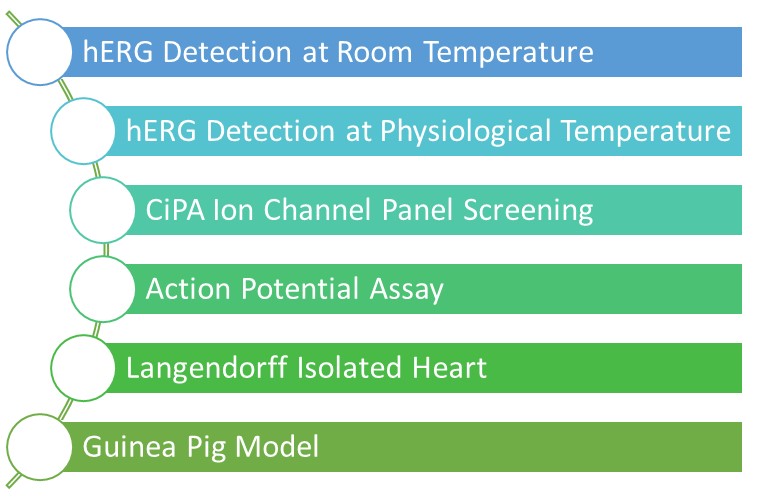
Download our brochure for Cardiac Safety Assessment to learn more about our integrated evaluation strategy.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.