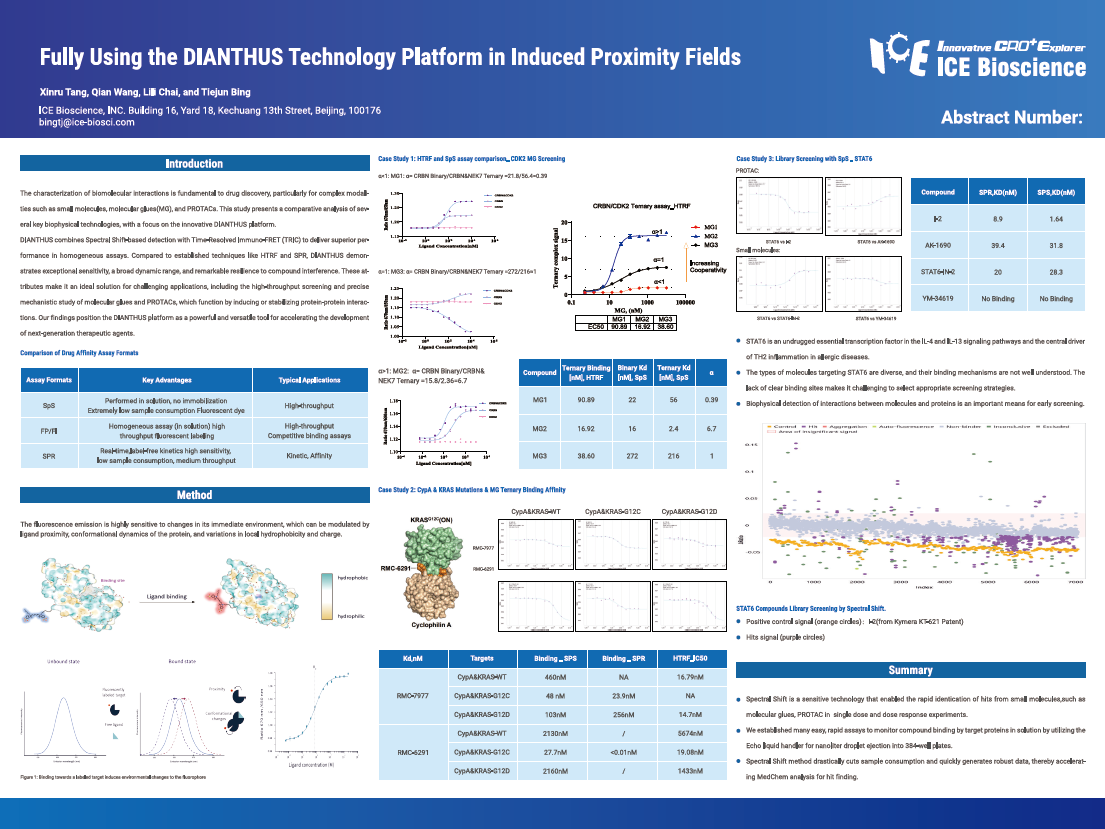
The characterization of biomolecular interactions is fundamental to drug discovery, particularly for complex modalities such as small molecules, molecular glues (MG), and PROTACs. This study presents a comparative analysis of several key biophysical technologies, with a focus on the innovative DIANTHUS platform.
DIANTHUS combines Spectral Shift-based detection with Time-Resolved Immuno-FRET (TRIC) to deliver superior performance in homogeneous assays. Compared to established techniques like HTRF and SPR, DIANTHUS demonstrates exceptional sensitivity, a broad dynamic range, and remarkable resilience to compound interference. These attributes make it an ideal solution for challenging applications, including the high-throughput screening and precise mechanistic study of molecular glues and PROTACs, which function by inducing or stabilizing protein-protein interactions. Our findings position the DIANTHUS platform as a powerful and versatile tool for accelerating the development of next-generation therapeutic agents.
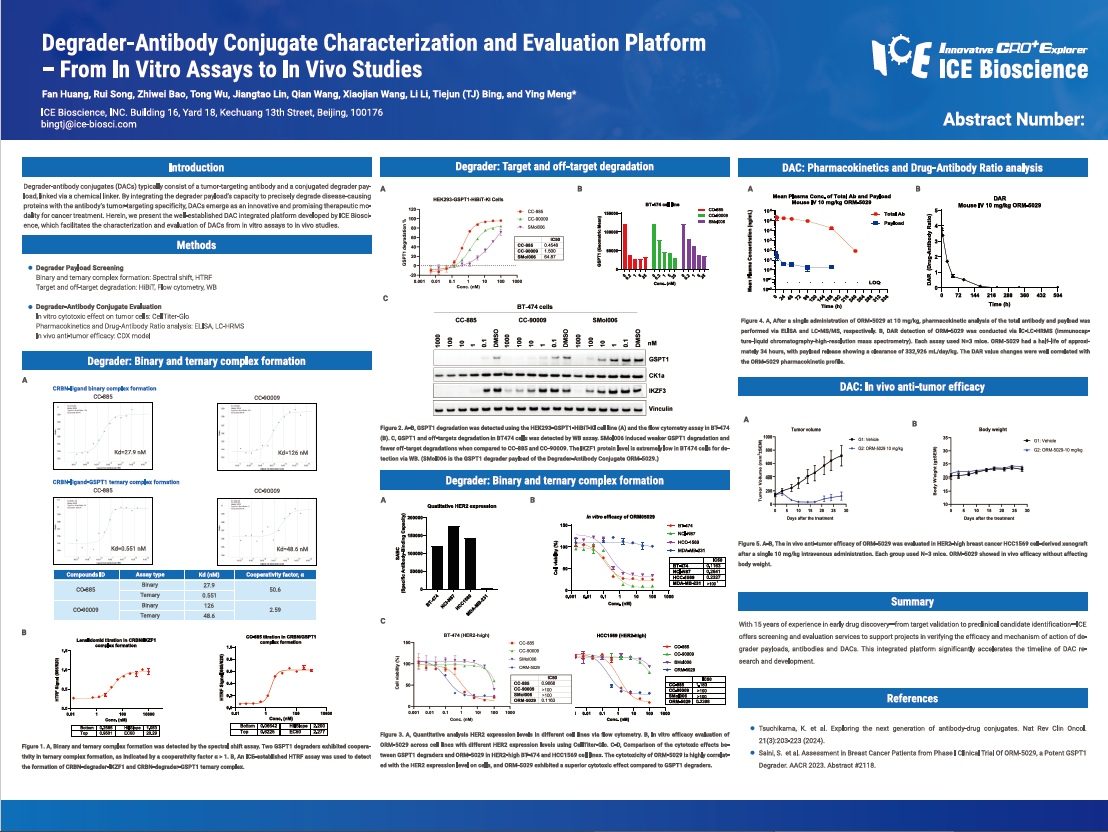
Degrader-antibody conjugates (DACs) typically consist of a tumor-targeting antibody and a conjugated degrader payload, linked via a chemical linker. By integrating the degrader payload’s capacity to precisely degrade disease-causing proteins with the antibody’s tumor-targeting specificity, DACs emerge as an innovative and promising therapeutic modality for cancer treatment. Herein, we present the well-established DAC integrated platform developed by ICE Bioscience, which facilitates the characterization and evaluation of DACs from in vitro assays to in vivo studies.
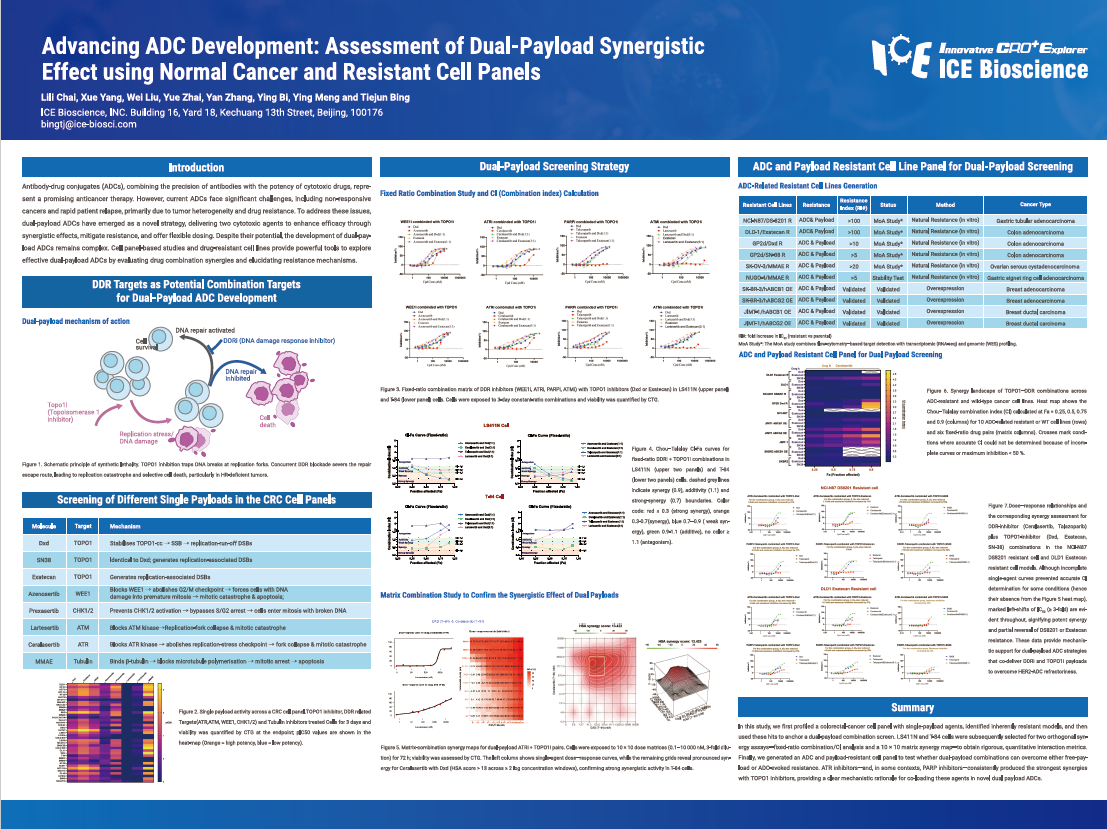
Antibody-drug conjugates (ADCs), combining the precision of antibodies with the potency of cytotoxic drugs, represent a promising anticancer therapy. However, current ADCs face significant challenges, including non-responsive cancers and rapid patient relapse, primarily due to tumor heterogeneity and drug resistance. To address these issues, dual-payload ADCs have emerged as a novel strategy, delivering two cytotoxic agents to enhance efficacy through synergistic effects, mitigate resistance, and offer flexible dosing. Despite their potential, the development of dual-payload ADCs remains complex. Cell panel-based studies and drug-resistant cell lines provide powerful tools to explore effective dual-payload ADCs by evaluating drug combination synergies and elucidating resistance mechanisms.
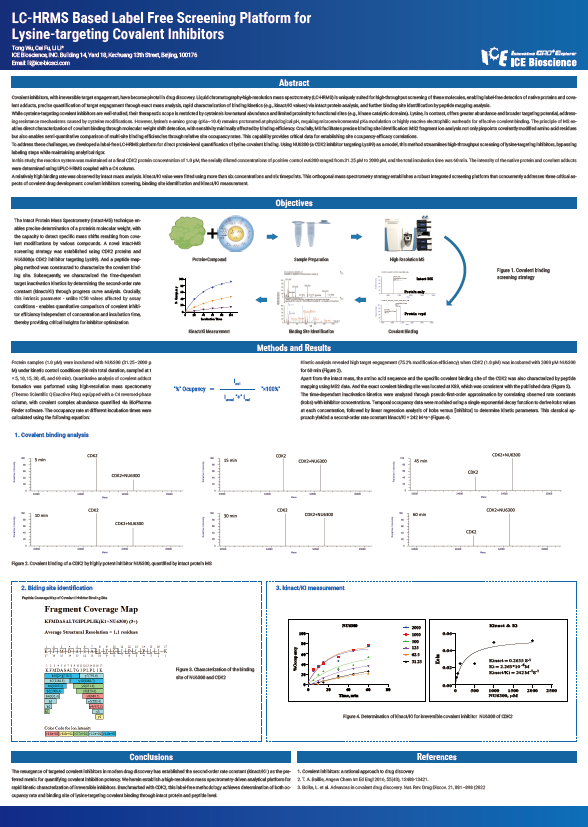
Covalent inhibitors, with irreversible target engagement, have become pivotal in drug discovery. Liquid chromatography-high-resolution mass spectrometry (LC-HRMS) is uniquely suited for high-throughput screening of these molecules, enabling label-free detection of native proteins and covalent adducts, precise quantification of target engagement through exact mass analysis, rapid characterization of binding kinetics (e.g., kinact/KI values) via intact protein analysis, and further binding site identification by peptide mapping analysis.
Address: Bldg 16, Yd 18, Kechuang 13th St, Etown, Tongzhou Dist, Beijing, 100176, China
Email: marketing@ice-biosci.com
Tel:+86-10-67809840
