Activation Assays of PBMCs (Peripheral Blood Mononuclear Cells) are essential immunological tools used to simulate in vivo environments, evaluate the impact of drugs on immune cells, and support the research and development of immunotherapeutic drugs. PBMCs consist of lymphocytes (e.g., T cells, B cells, NK cells), monocytes, and dendritic cells, all of which play critical roles in immune response and regulation.
Typical Steps in PBMC Activation Assays:
1. Cell Isolation: PBMCs are isolated from peripheral blood using methods such as density gradient centrifugation.
2. Cell Culture and Stimulation: Isolated PBMCs are cultured in vitro and activated using stimuli such as anti-CD3/CD28 antibodies, plant lectins (e.g., PHA), Staphylococcus aureus enterotoxin B (SEB), interleukin-27 (IL-27), or phorbol myristate acetate (PMA) combined with ionomycin to induce T cell activation.
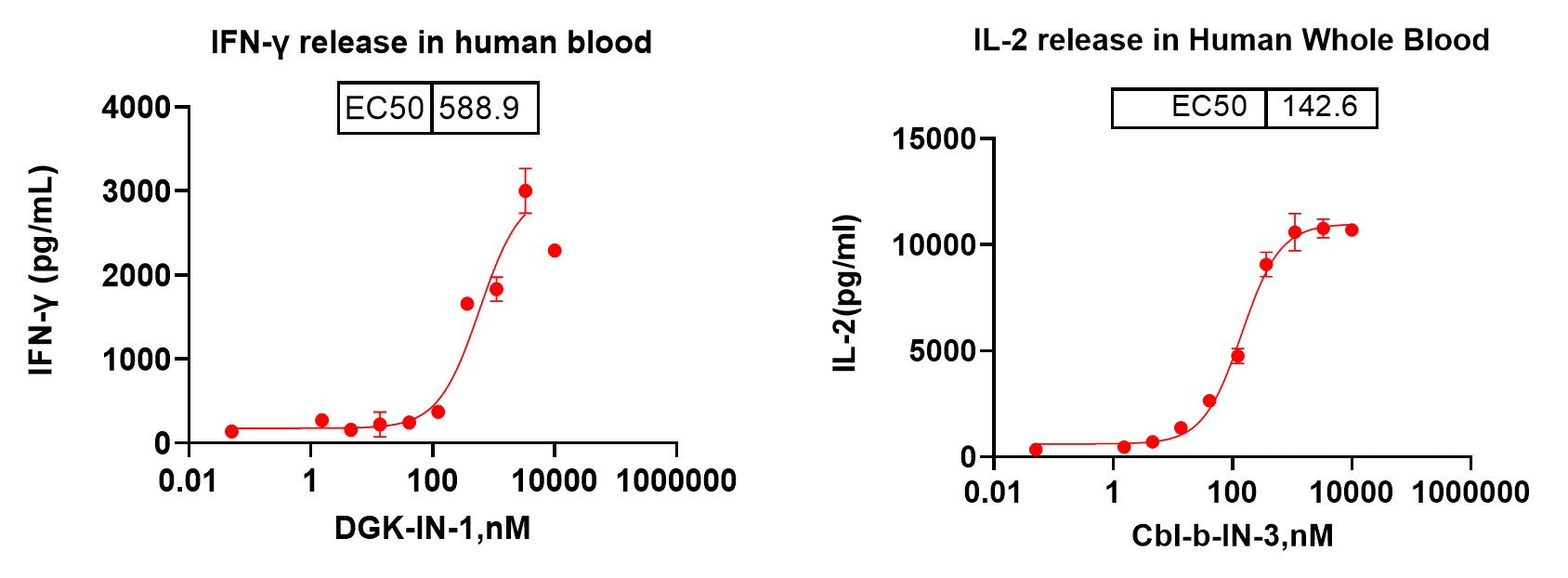
3. Activation Assessment: PBMC activation is assessed by examining cell surface markers (e.g., CD69 expression), measuring cell proliferation, and analyzing cytokine secretion (e.g., IFN-γ).
4. Data Analysis: Techniques such as flow cytometry, ELISA, and CellTiter-Glo luminescence assays are employed to analyze data, providing insights into the drug's effect on PBMC activation.
These assays are crucial for understanding immune system function, evaluating the efficacy of immunotherapeutic drugs, and identifying novel drug targets. Researchers can assess drug-induced changes in PBMC proliferation, apoptosis, and cytotoxicity, allowing for preliminary evaluation of a drug's efficacy and safety profile.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.