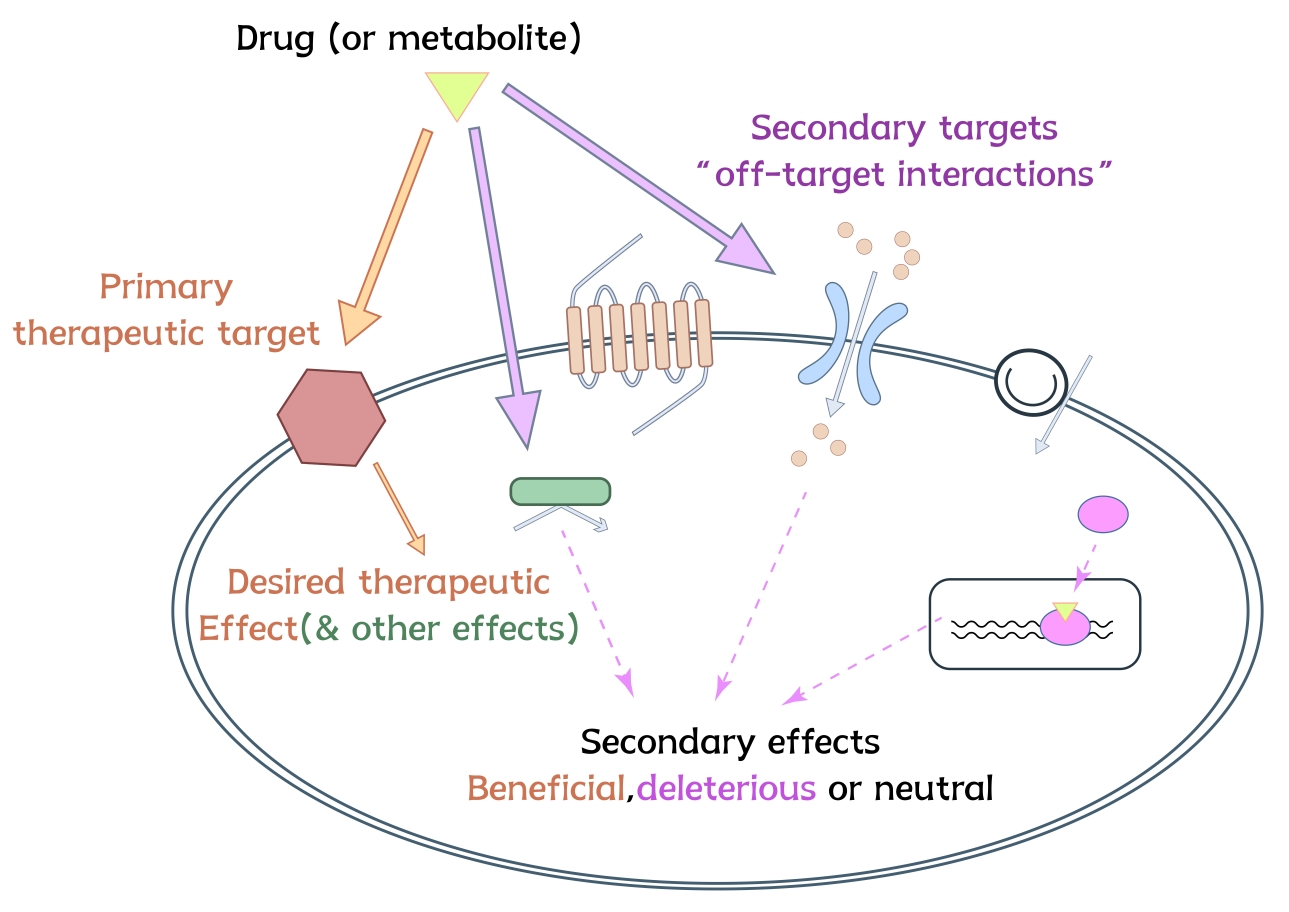
In vitro safety pharmacology and secondary pharmacology both aim to de-risk drug candidates by identifying potential off-target effects before clinical development. Secondary pharmacology focuses on evaluating a compound’s activity at non-primary targets that may lead to adverse drug reactions, using broad in vitro panels that reflect key physiological systems. As regulatory expectations rise, in vitro profiling has become a critical component of early drug safety assessment—providing mechanistic insights, supporting candidate selection, and reducing late-stage attrition.
The theoretical foundation for safety panel services is based on the publication "Reducing safety-related drug attrition: The use of in vitro pharmacological profiling" from Nature Reviews Drug Discovery (2012). This publication highlights the importance of early identification of off-target activities to reduce late-stage attrition in drug development. Furthermore, "The state of the art in secondary pharmacology and its impact on the safety of new medicines" from Nature Reviews Drug Discovery (2024) summarizes insights from 18 major pharmaceutical companies, highlighting the limitations of binding-only assays and supports the adoption of functional, dose–response–based profiling to better predict off-target risks and inform safer drug development decisions.
✅ Binding ≠ true activity — often overestimates hits
✅ Functional assays reduce false positives and unnecessary follow-up
✅ Better at detecting allosteric or agonist effects, especially for ion channels
✅ Relying on binding alone risks missing key liabilities or rejecting good compounds
Customize Your In Vitro Functional Safety Panels
ICE Bioscience leads in Safety Pharmacology services. Tailor your own panels to meet your specific drug discovery needs. Our experts and a vast portfolio of in vitro assays are at your service. Let us drive your drug discovery program forward.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.