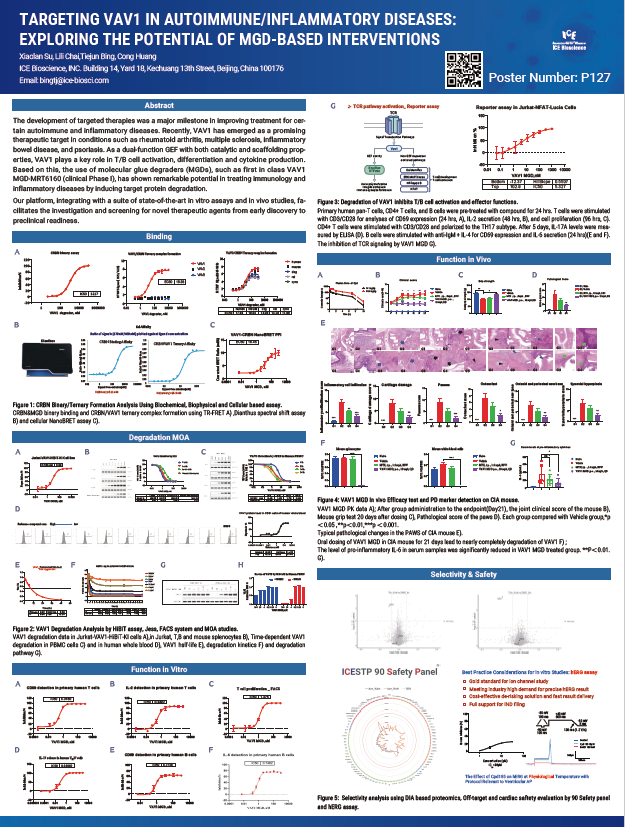
The development of targeted therapies was a major milestone in improving treatment for certain autoimmune and inflammatory diseases. Recently, VAV1 has emerged as a promising therapeutic target in conditions such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and psoriasis. As a dual-function GEF with both catalytic and scaffolding properties, VAV1 plays a key role in T/B cell activation, differentiation and cytokine production. Based on this, the use of molecular glue degraders (MGDs), such as first in class VAV1 MGD-MRT6160 (clinical Phase I), has shown remarkable potential in treating immunology and inflammatory diseases by inducing target protein degradation.
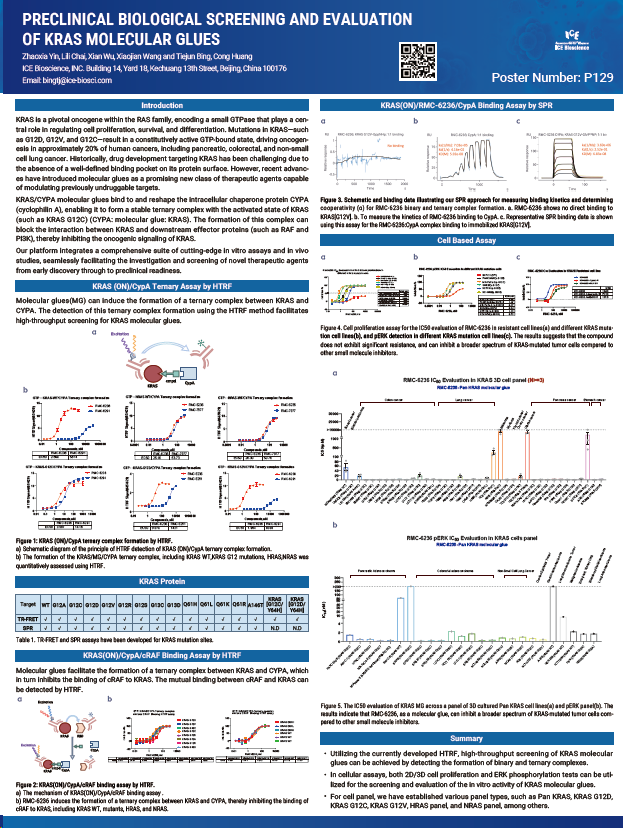
KRAS is a pivotal oncogene within the RAS family, encoding a small GTPase that plays a central role in regulating cell proliferation, survival, and differentiation. Mutations in KRAS—such as G12D, G12V, and G12—result in a constitutively active GTP-bound state, driving oncogenesis in approximately 20% of human cancers, including pancreatic, colorectal, and non-small cell lung cancer. Historically, drug development targeting KRAS has been challenging due to the absence of a well-defined binding pocket on its protein surface. However, recent advances have introduced molecular glues as a promising new class of therapeutic agents capable of modulating previously undruggable targets.

In pharmaceutical development, early assessment of drug candidate safety is critical to mitigate post-market withdrawals and reduce risks. Approximately 75% of adverse drug reactions (ADRs) are dose-dependent (Type A), often linked to off-target interactions. To address this, we have introduced ICESTP SAFETYPANELTM 44, a product comprising 44 screening models designed to assess target safety across critical areas such as the central nervous system, cardiovascular system, metabolism, and immunity. This panel has already successfully screened over 700 different types of compounds. Building on this initial product, we have further developed two new products: ICESTP SAFETYPANELTM 77 and ICESTP SAFETYPANETM PLUS, as shown in Figure 1, which are cutting-edge functional safety assessment panels designed to revolutionise drug development. By evaluating the agonist or antagonist effects of compounds on these targets through functional activity screening, we assess compound safety and provide reference criteria for later stages of drug development. We tested pegolide and three antidepressants and two anticancer compounds on the ICESTP SAFETYPANELTM 77. The results demonstrated that our panel provides a competitive advantage by enabling the early identification of potential off-target ADRs and supporting informed decision-making, ultimately driving safer and more effective drug development.

Drug resistance is a major challenge in cancer therapy, often causing relapse after initial successful treatment. Exploring resistance mechanisms and developing new treatments are key goals in oncology. Several promising targets for anticancer drug development have been identified or are under active investigation. For instance, KRAS mutations are prevalent in pancreatic, colorectal, and lung cancers, driving uncontrolled cell proliferation. DNA damage repair (DDR) mechanisms normally maintain genomic stability by repairing DNA damage, but defects in DDR pathways can make cancer cells more susceptible to targeted therapies like PARP inhibitors. EGFR resistance is frequently observed in clinical settings, and the emerging field of antibody-drug conjugate (ADC) therapies is also encountering drug resistance challenges. Drug-resistant cell lines are valuable tools for studying these resistance mechanisms and discovering novel therapies.