Our Killing Assay services are designed to provide a comprehensive evaluation of the cytotoxic effects of various immune cells, including T cells, Peripheral Blood Mononuclear Cells (PBMCs), and Natural Killer (NK) cells, on target cells. These assays are crucial for understanding the mechanisms of immune-mediated cell killing, which is fundamental in cancer research, infectious disease studies, and the development of immunotherapies.
Lead Compound Identification: Screen potential drug candidates for their ability to enhance or inhibit immune cell-mediated cytotoxicity, identifying promising compounds early in the drug development process.
Mechanism of Action (MoA) Insights: Understand how drugs influence immune cell activity, such as modulating T cell or NK cell functions, to elucidate the mechanisms driving their therapeutic effects.
Immunotherapy Optimization: Fine-tune and evaluate the efficacy of immunotherapies, including CAR-T cells, checkpoint inhibitors, and bispecific antibodies, by assessing their impact on immune cell-mediated killing.
Safety and Toxicity Evaluation: Determine the safety profile of drug candidates by assessing off-target effects on healthy cells, ensuring that treatments specifically target diseased cells.
Biomarker Discovery: Identify predictive and prognostic biomarkers from assay data to support the development of personalized therapies and enhance patient stratification in clinical trials.
Animal-Derived T Cells: Incorporate T cells from animal models, such as mouse T cells, to study cross-species immunological responses or preclinical models.
Human T Cells: Use patient-derived (autologous) T cells for personalized assessments or T cells from healthy donors (allogeneic) for standardized testing.
Engineered T Cells: Incorporate genetically modified T cells, such as CAR-T cells, to evaluate specific immune responses.
T Cell Subsets: Isolate and study specific T cell subsets (e.g., CD8+ cytotoxic T cells, CD4+ helper T cells).
Tumor Cell Lines: Assess T cell cytotoxicity against various cancer cell lines.
Primary Tumor Cells: Use patient-derived tumor cells for more clinically relevant results.
Custom Targets: Specify particular cell types or engineered target cells to match your research needs.
Flow Cytometry: Quantify cell death, apoptosis, and immune cell activation with high precision.
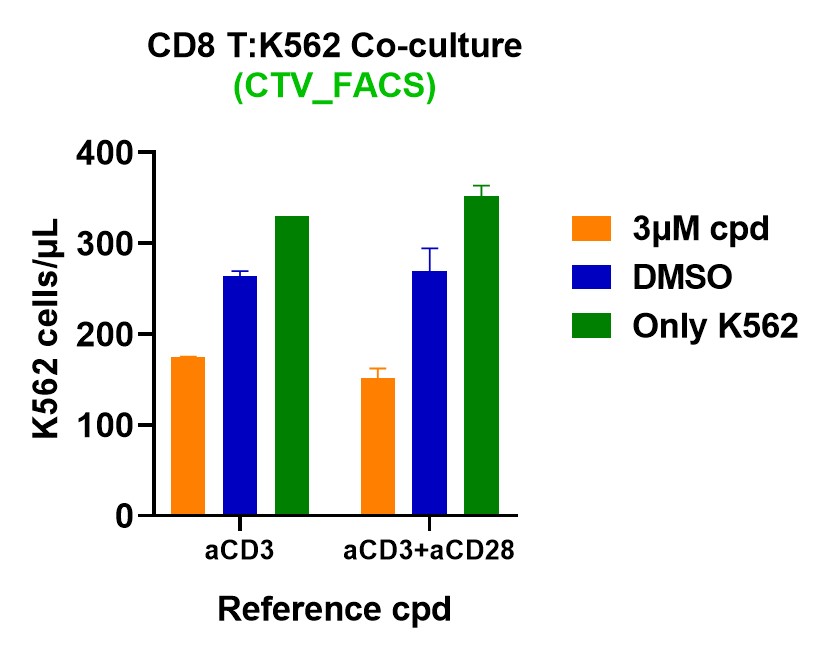
Incucyte Live-Cell Imaging: Monitor real-time cell killing and proliferation.
Luminescence Assays: Measure ATP levels as an indicator of cell viability.
Cytokine Analysis: Assess cytokine release (e.g., IFN-γ, TNF-α) to evaluate T cell activation and function.
Our PBMC Killing Assay service is designed to provide a comprehensive evaluation of the cytotoxic activity of Peripheral Blood Mononuclear Cells (PBMCs) against target cells. PBMCs, which include a diverse mixture of immune cells such as T cells, B cells, NK cells, and monocytes, offer a more physiologically relevant model for studying immune responses. This assay is crucial for understanding how different immune cell populations collectively contribute to the elimination of cancerous or infected cells, making it an essential tool in immunotherapy research and drug development.
Flow Cytometry: Quantify the killing efficiency and activation of specific immune cell subsets within PBMCs.
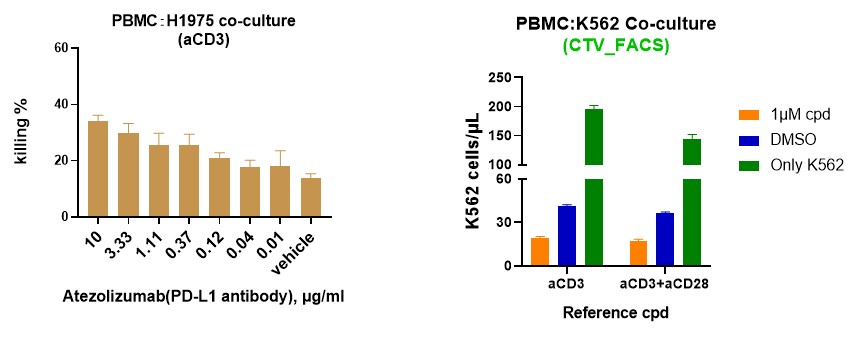
Incucyte Live-Cell Imaging: Monitor real-time cytotoxicity and immune cell interactions over time.
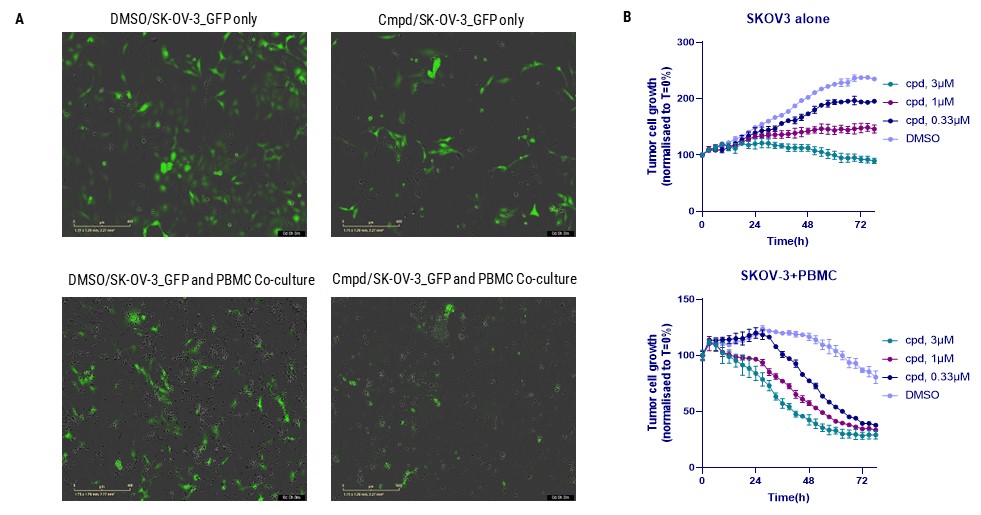
Figure. Evaluation of METTL3 inhibitor (METTL3i) efficacy in enhancing PBMC-mediated tumor cell killing using the Incucyte system. (A) It displays images of SKOV-3 ovarian cancer cells alone and in a co-culture system with PBMCs, both untreated and treated with METTL3i. (B) We quantitatively analyze tumor cell viability over 72 hours. The upper graph shows the impact of METTL3i alone on SKOV-3 cells, and the lower graph illustrates the enhanced cytotoxic effect of PBMCs in combination with METTL3i on tumor viability. Green fluorescence marks live cells, capturing the dynamic response and demonstrating the compound's dose-dependent enhancement of PBMC-mediated cytotoxicity against cancer cells.
Our NK Cell Killing Assay service is designed to evaluate the natural cytotoxicity of Natural Killer (NK) cells, as well as their ability to mediate Antibody-Dependent Cellular Cytotoxicity (ADCC). NK cells are crucial components of the innate immune system, known for their ability to directly target and eliminate virally infected and tumor cells without prior sensitization. This assay is essential for understanding NK cell function and for developing therapeutic strategies that leverage NK cells in cancer immunotherapy and infectious disease treatment.
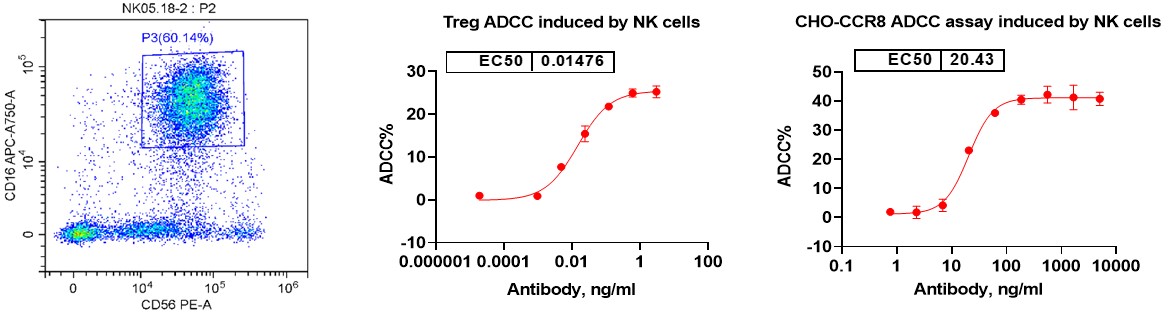
Direct NK Cell Cytotoxicity: Assess the intrinsic ability of NK cells to recognize and kill target cells, such as tumor or virally infected cells. This aspect of the assay provides insights into the natural cytotoxic mechanisms of NK cells, including the release of perforin and granzyme.
ADCC (Antibody-Dependent Cellular Cytotoxicity): Evaluate the enhanced cytotoxicity of NK cells when antibodies are introduced to target cells. In the ADCC component of the assay, antibodies bind to specific antigens on the surface of target cells, marking them for destruction. NK cells, through their Fcγ receptors (e.g., CD16), recognize the Fc region of these antibodies and are activated to kill the target cells more efficiently.
High-Content Imaging: Utilize high-content imaging to obtain detailed, multi-parametric data on cell killing, morphology changes, and NK cell-target cell interactions.
Incucyte Live-Cell Imaging: Monitor real-time cytotoxicity and visualize NK cell interactions with target cells over time, providing dynamic insights into cell behavior.
Luminescence Assays: Measure ATP levels as an indicator of cell viability and NK cell-mediated killing, offering a straightforward and quantifiable readout.
Flow Cytometry: Analyze NK cell phenotype and quantify cytotoxicity by measuring the expression of key surface markers such as CD56 and CD16, which identify NK cell populations. Additionally, assess NK cell-mediated killing efficiency through dose-response curves that track the extent of target cell death, providing detailed insights into both direct cytotoxicity and ADCC activity.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.