Nano Differential Scanning Fluorimetry (NanoDSF) is a powerful, label-free technique used to analyze the thermal stability and folding behavior of biomolecules, such as proteins, peptides, and nucleic acids. The method relies on measuring the intrinsic fluorescence of aromatic amino acids, primarily tryptophan and tyrosine, as the protein undergoes thermal unfolding. The Prometheus system from NanoTemper Technologies, employed in our service, provides real-time, high-resolution data on protein stability by detecting subtle changes in fluorescence intensity as a function of temperature. This allows for precise determination of key thermodynamic properties, including the melting temperature (Tm) and the transition midpoint, offering valuable insights into how a protein behaves under different conditions.
NanoDSF is widely applied in drug discovery and development, including studies of protein stability, formulation optimization, and protein-ligand interactions. It is particularly valuable for evaluating the thermal stability of therapeutic proteins, as well as assessing the effects of ligand binding on protein structure. Additionally, NanoDSF is used for protein-protein interaction studies and biomarker validation.
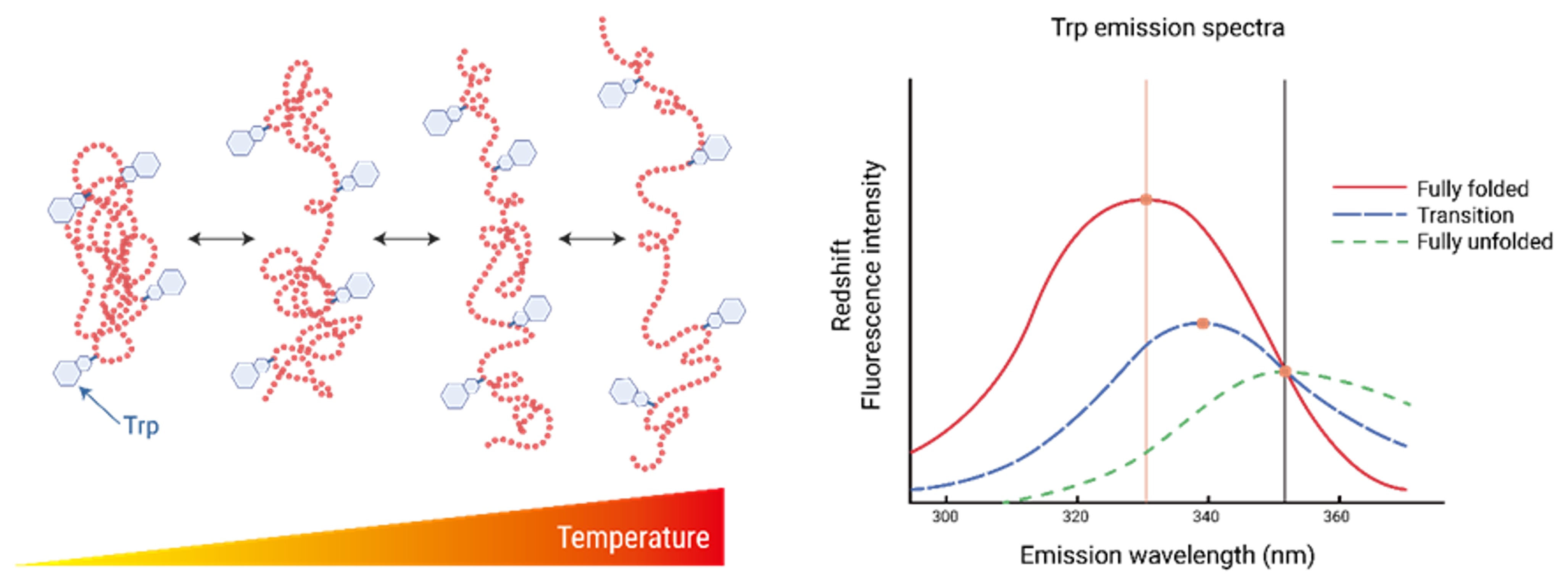
Figure. NanoDSF Assay Principle. Proteins contain tryptophan residues that absorb light at 280 nm, and their fluorescence emission properties change based on the surrounding chemical environment. In a folded, native state, tryptophan residues emit fluorescence with a peak around 330 nm. However, when proteins unfold and expose their hydrophobic regions to the solution, the emission properties shift, with a peak fluorescence around 350 nm. These conformational changes are influenced by factors like temperature or other environmental conditions, resulting in alterations in fluorescence intensity and emission wavelength. By monitoring these changes, key parameters such as the melting temperature (Tm) of the protein can be determined.
Advanced Detection Technology:NanoDSF detects protein unfolding by measuring the autofluorescence changes of tryptophan and tyrosine, eliminating the need for dyes. This allows for accurate detection of thermal denaturation under native conditions without interference.
High Data Quality:NanoDSF ensures precise detection across various buffer conditions with a detection range from 5 μg/mL to 250 mg/mL. It is suitable for proteins of all sizes, including enzymes, antibodies, ADCs, and membrane proteins, and provides reliable data even with sample aggregation.
High-Throughput Capability:The system can simultaneously measure up to 48 samples, improving efficiency for high-throughput screening.
Low Sample Volume:Only 10 μL per sample is required, conserving valuable sample resources.
At ICE Bioscience, we offer both ready-to-use NanoDSF assays and customized NanoDSF services, with our assay catalog constantly expanding.
Our custom services allow us to tailor assays to your specific research needs and targets, ensuring the highest level of accuracy and relevance. Meanwhile, our extensive range of pre-developed assays provides immediate access to validated, reliable assays, saving you valuable time and resources.
This combination of flexible custom solutions and ready-to-use options ensures that ICE Bioscience can effectively support the diverse needs of the pharmaceutical and biotechnology industries.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.