☑️ Identification of Unknown Small Molecules: Expertise in using non-targeted LC-HRMS methods to identify unknown small molecules containing payloads released from ADCs and their metabolites.
☑️ Comprehensive ADC Stability Studies: Ability to thoroughly investigate ADC stability, from lysosomal stability to payload release in biological matrices, ADC concentration changes, and DAR (Drug-to-Antibody Ratio) variations.
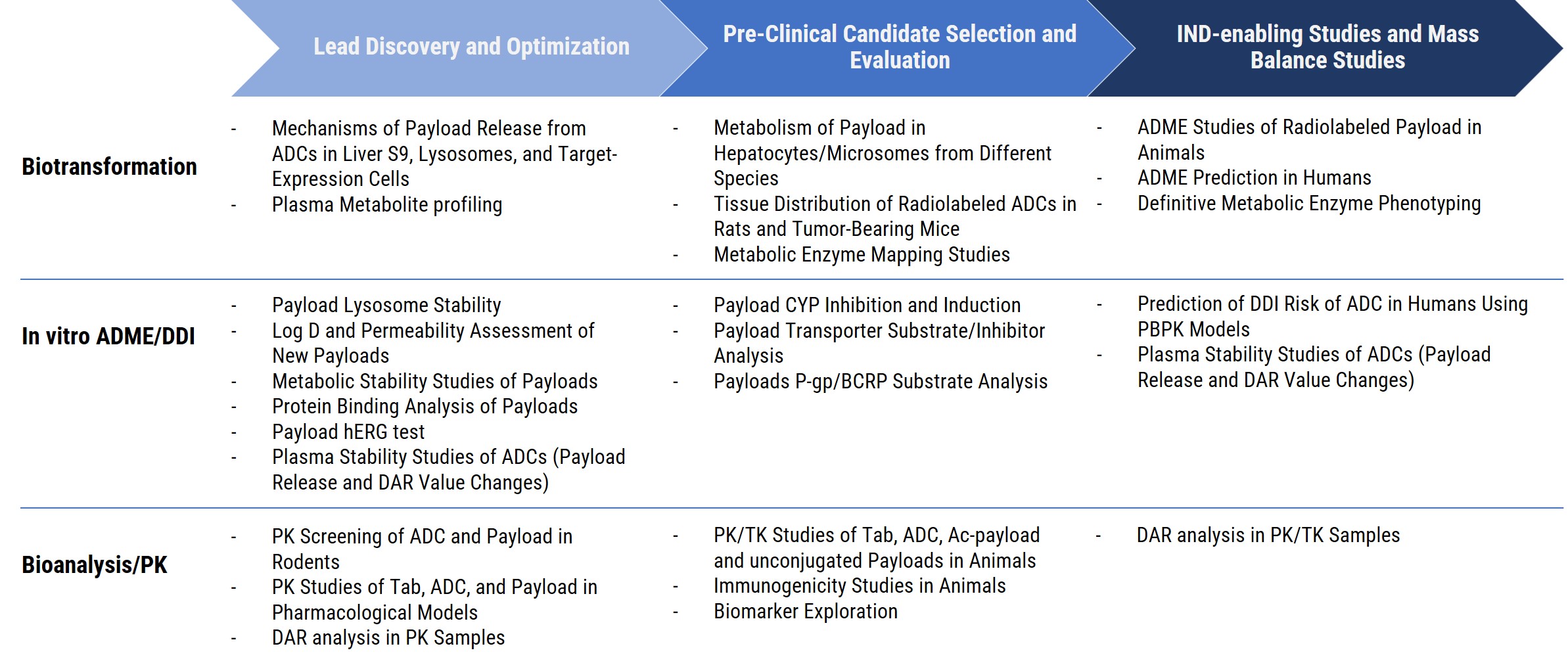
☑️ Our DMPK platform offers exceptional flexibility and extensive experience across multiple modalities, including payloads, small molecules, peptides, biologics, and Antibody-Drug Conjugates (ADCs), providing tailored solutions to meet the diverse needs of drug development projects.
☑️ Complete Transporter Evaluation Platform
☑️ Experience in Animal Radioisotope ADME Studies
☑️ Experience in Bioanalytical Assay Development
MINI CASE STUDIES
The LC-HRMS analysis provided detailed insights into the metabolites formed from the uncleavable ADC. The identification of various metabolic forms of the payload, reveals the complexity of ADC metabolism and highlights the importance of thorough metabolic characterization in ADC development. This knowledge aids in the optimization of ADC design to improve stability, efficacy, and safety. Contact us for more detailed case study data.
For DS-8201, a HER2-targeting ADC, this evaluation helps in understanding how the Drug-to-Antibody Ratio (DAR) changes over time and how stable the payload and antibody components are in plasma. The findings highlight the importance of early stability assessments in ADC development, particularly for optimizing the linker and overall molecular stability. By leveraging our comprehensive stability study platform, including free small molecule toxins, conjugated small molecule toxins, conjugated antibodies, total antibodies, and DAR values, we can guide the optimization of ADC molecules to improve their therapeutic potential. Contact us for more detailed case study data.
We have developed a robust LC-MS-based quantitative method for analyzing DS-8201 (an Antibody-Drug Conjugate) and Trastuzumab (Naked antibody). Our LC-MS method provides results consistent with those obtained using ELISA, effectively meeting the diverse needs for biologics quantification in various scenarios. This comparison demonstrates the reliability and accuracy of our LC-MS method, making it a valuable tool for comprehensive ADC and antibody analysis. Contact us for more detailed case study data.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.