Spectral Shift is a technique used to study molecular interactions. During detection, one of the molecules is labeled with a fluorophore. When the fluorescently labeled molecule interacts with a ligand, changes occur in the chemical environment around the fluorophore, causing a shift in its fluorescence emission spectrum. These spectral changes are monitored to determine the occurrence and extent of binding between the molecule and the ligand.
As an emerging technique for detection of molecular interactions, spectral shift technology can provide high-quality data, sensitively detect more true binding molecules, and provide reliable sample detection results with less assay optimization.
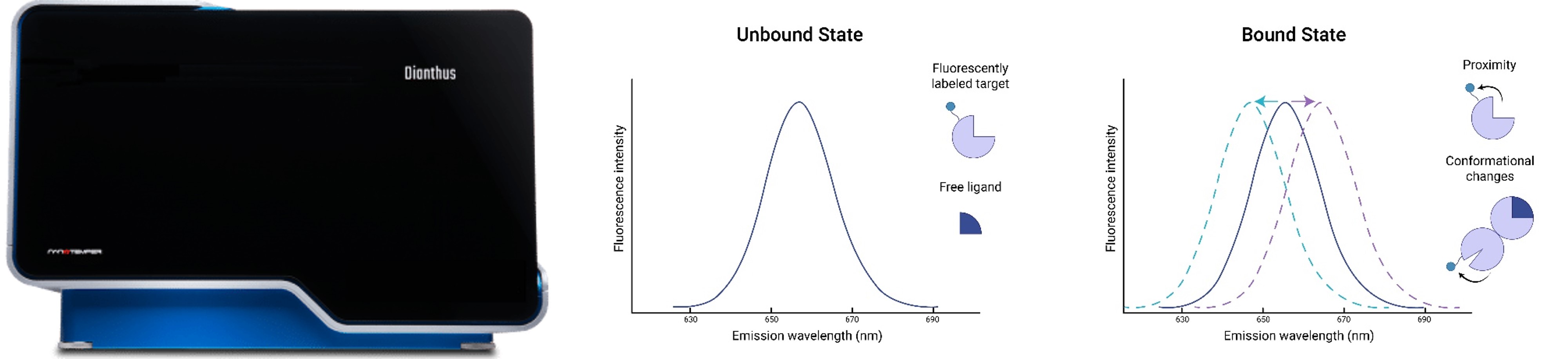
Nanotemper Dianthus and assay principle. The spectral shift technique is based on the principle that when a target molecule interacts with a ligand, the chemical environment around the fluorophore attached to the target molecule changes. This interaction causes a subtle shift in the emission wavelength of the fluorophore under isothermal conditions. In spectral shift measurements, the fluorescence ratio is determined by simultaneously recording the emission fluorescence at two pre-selected wavelengths, enabling sensitive detection of binding events.
☑️ In-House Proteins: Utilizing our highly active, in-house produced proteins, we offer a broad range of biophysical assays tailored to diverse research needs.
☑️ Wide Range of Applications: Our platform supports screening of small molecules or fragments, affinity analysis for hit validation and lead optimization, structure-activity relationship (SAR) studies. It is also suitable for characterizing binary and ternary complexes of protein degraders and studying protein-protein interactions.
☑️ High Sensitivity: Leveraging our state-of-the-art Dianthus system, we detect even sub-nanometer spectral shifts at maximum emission wavelengths, ensuring highly precise binding affinity measurements.
☑️ High Throughput: Capable of completing 384-well microplate detection in just 33 minutes and determining Kd values within 1 minute per sample, meeting the stringent demands of high-throughput screening.
☑️ High Flexibility: A microplate-based, microfluidic-free affinity screening platform that operates entirely in solution. Assays are independent of molecular weight variations, making this platform ideal for tackling challenging screening projects.
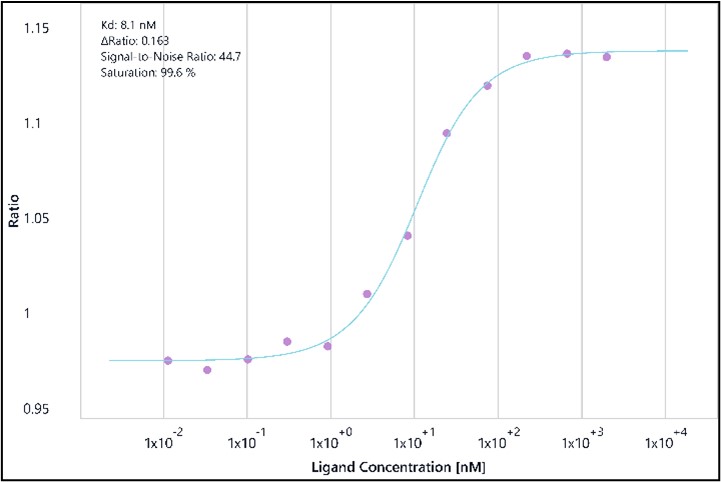
Binding affinity assessment of MZ1 (a PROTAC) to BRD2 (BD2) using Spectral Shift technology. The dose-response curve shows the binding interaction between MZ1 and BRD2, yielding a dissociation constant (Kd) of 8.1 nM. Key metrics include an S/B ratio of 0.163, a signal-to-noise ratio of 44.7, and a saturation level of 99.6%, demonstrating a high-affinity binding interaction. BRD2, known for its role in recognizing acetylated lysines on histones, participates in chromatin remodeling, transcriptional regulation, and cellular processes such as proliferation and apoptosis.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.