Our comprehensive suite of ADCC (Antibody-Dependent Cellular Cytotoxicity), ADCP (Antibody-Dependent Cellular Phagocytosis), and CDC (Complement-Dependent Cytotoxicity) assay services are designed to evaluate the mechanisms by which therapeutic antibodies and other immunomodulatory agents mediate immune responses against target cells, such as cancer cells or pathogens. These assays are essential in the development and optimization of antibody-based therapies, providing critical insights into their efficacy and mechanism of action.
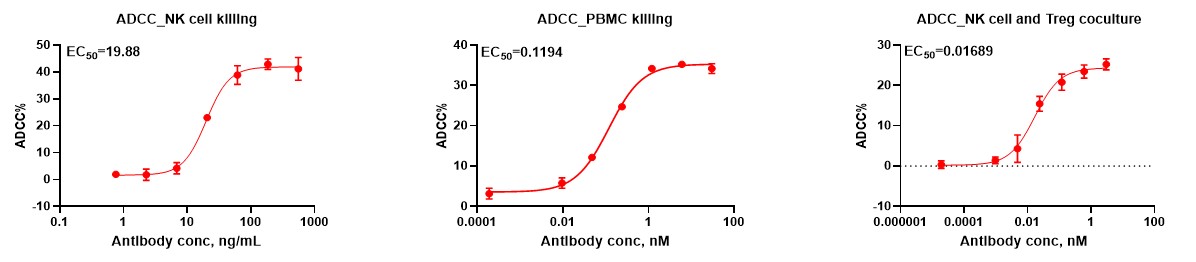
ADCC assays measure the ability of immune effector cells, such as NK cells, to recognize and kill antibody-coated target cells. This mechanism is critical for the action of many therapeutic antibodies, particularly in cancer immunotherapy.
Key Features:
Effector Cells: Typically involves NK cells as primary effector cells, but can also include macrophages, neutrophils, and other Fc receptor-expressing cells.
Target Cells: Includes a wide range of tumor cell lines, virally infected cells, or engineered cells expressing specific antigens targeted by therapeutic antibodies.
Antibody Interaction: Utilizes therapeutic antibodies or monoclonal antibodies that specifically bind to antigens on the target cells, triggering effector cell activation through Fcγ receptors.
Assay Readouts: Cytotoxicity is measured using flow cytometry to assess the extent of target cell death, luminescence-based assays for ATP content (indicative of cell viability), or high-content imaging to visualize and quantify cell killing.
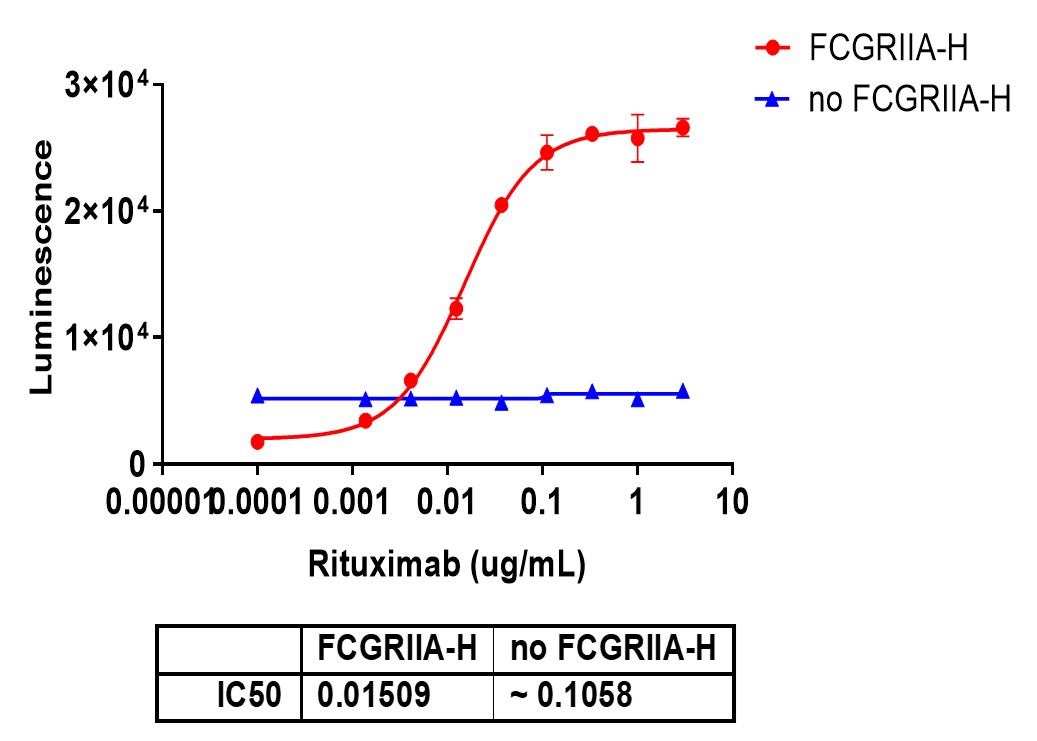
ADCP assays evaluate how effectively antibodies promote the phagocytosis of target cells by immune cells such as macrophages. This process is important for the clearance of antibody-coated pathogens, dead cells, or cancer cells from the body.
Key Features:
Effector Cells: Primarily uses monocytes, macrophages, and dendritic cells, which express Fcγ receptors like FcγRIIa (CD32a), FcγRI (CD64), and FcγRIIIa (CD16a).
Phagocytic Activity: Assesses the ability of these cells to engulf and digest antibody-opsonized target cells, a critical mechanism in immune defense and therapeutic antibody function.
Target Cells: Includes tumor cells, infected cells, or particles labeled with specific antibodies for phagocytosis.
Assay Readouts: Depending on the approach, the readouts can be direct (measuring phagocytosis via flow cytometry or high-content imaging) or indirect (measuring reporter gene activity as a proxy for Fcγ receptor engagement and subsequent signaling).
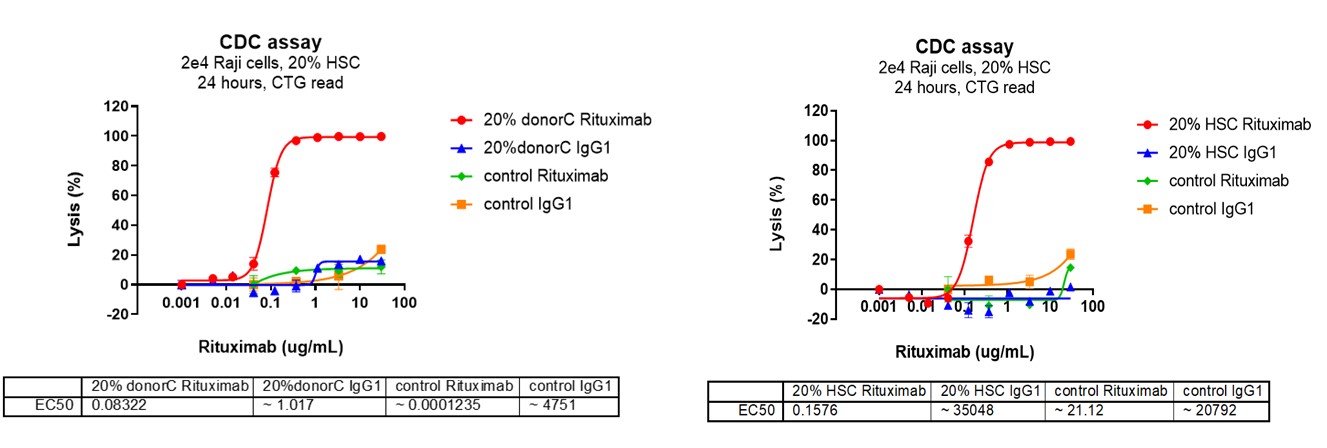
CDC assays measure the ability of antibodies to activate the complement system, leading to the lysis of target cells. This mechanism is essential for the action of certain therapeutic antibodies, particularly in hematological cancers.
Key Features:
Complement Source: Utilizes human serum or plasma as a source of complement proteins to initiate the complement cascade upon antibody binding to target cells.
Target Cells: Focuses on cell lines or primary cells that express the target antigen for the therapeutic antibody, such as CD20 on B cells in lymphoma.
Membrane Attack Complex (MAC) Formation: The endpoint of the complement cascade, where the MAC forms pores in the target cell membrane, leading to cell lysis.
Assay Readouts: Cell lysis is detected using assays that measure the release of intracellular components (e.g., lactate dehydrogenase), flow cytometry to assess cell death, or luminescence-based assays to evaluate the loss of cell viability.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.