Our MST-TRIC service combines the principles of MicroScale Thermophoresis (MST) with advanced Temperature-Related Intensity Change (TRIC) technology, delivering precise and high-sensitivity binding analysis. With TRIC's enhanced capabilities for equilibrium-based measurements, we provide an innovative solution for studying molecular interactions under physiologically relevant conditions. Temperature-Related Intensity Change (TRIC) is a solution-based biophysical technique for studying molecular interactions. By detecting fluorescence changes induced by localized temperature shifts, TRIC enables the accurate determination of binding affinity (Kd) for various target-ligand systems.
MST-TRIC measures fluorescence intensity changes resulting from a brief, localized temperature increase induced by an infrared laser. Binding events alter the fluorescent environment, and these changes are amplified by the temperature shift, providing highly sensitive binding detection.
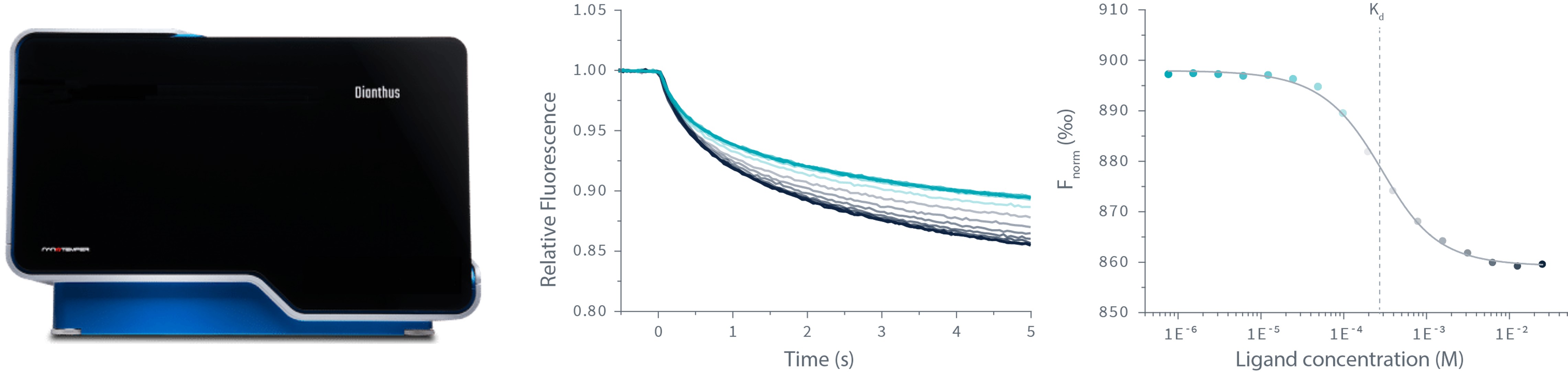
Nanotemper Dianthus and assay principle. The MST-TRIC assay is based on the principle that when fluorescently labeled molecules bind to their ligands, the chemical environment of the fluorophore changes, resulting in a shift in fluorescence intensity. During the measurement, the sample is briefly heated by an infrared laser, which raises the temperature and amplifies the changes in fluorescence intensity associated with ligand binding. By monitoring these temperature-induced fluctuations in fluorescence, the interaction between biomolecules can be assessed.
In-House Proteins: Utilizes highly active in-house produced proteins, enabling a wide range of biophysical assays.
Versatile Applications: Supports single-dose screening of small molecules or fragments, affinity analysis for hit validation, hit-to-lead optimization, and classification post-high-throughput screening. Suitable for characterizing binary and ternary complexes, including protein degraders, and for studying protein-protein interactions critical to cellular processes such as signaling pathways.
High Sensitivity: The advanced Dianthus system detects affinities at the picomolar (pM) level, providing precise and reliable measurements.
High Throughput: Enables rapid detection using 384-well microplates, completing assays in as little as 73 minutes to meet the demands of high-throughput screening.
High Flexibility: A microplate-based, microfluidic-free affinity screening platform that operates entirely in solution and is independent of molecular weight variations, making it highly suitable for challenging screening projects.
BRD2 protein specifically binds to histone acetylated lysine and participates in gene transcription regulation, chromatin remodeling, cell proliferation, apoptosis, and other biological activities. A fragment-based screening of BRD2 (BD2) was performed using a 324-fragment library with MST-TRIC. The binding of hits to BRD2 (BD2) was confirmed by measuring the affinity of the hits to BRD2 (BD2).
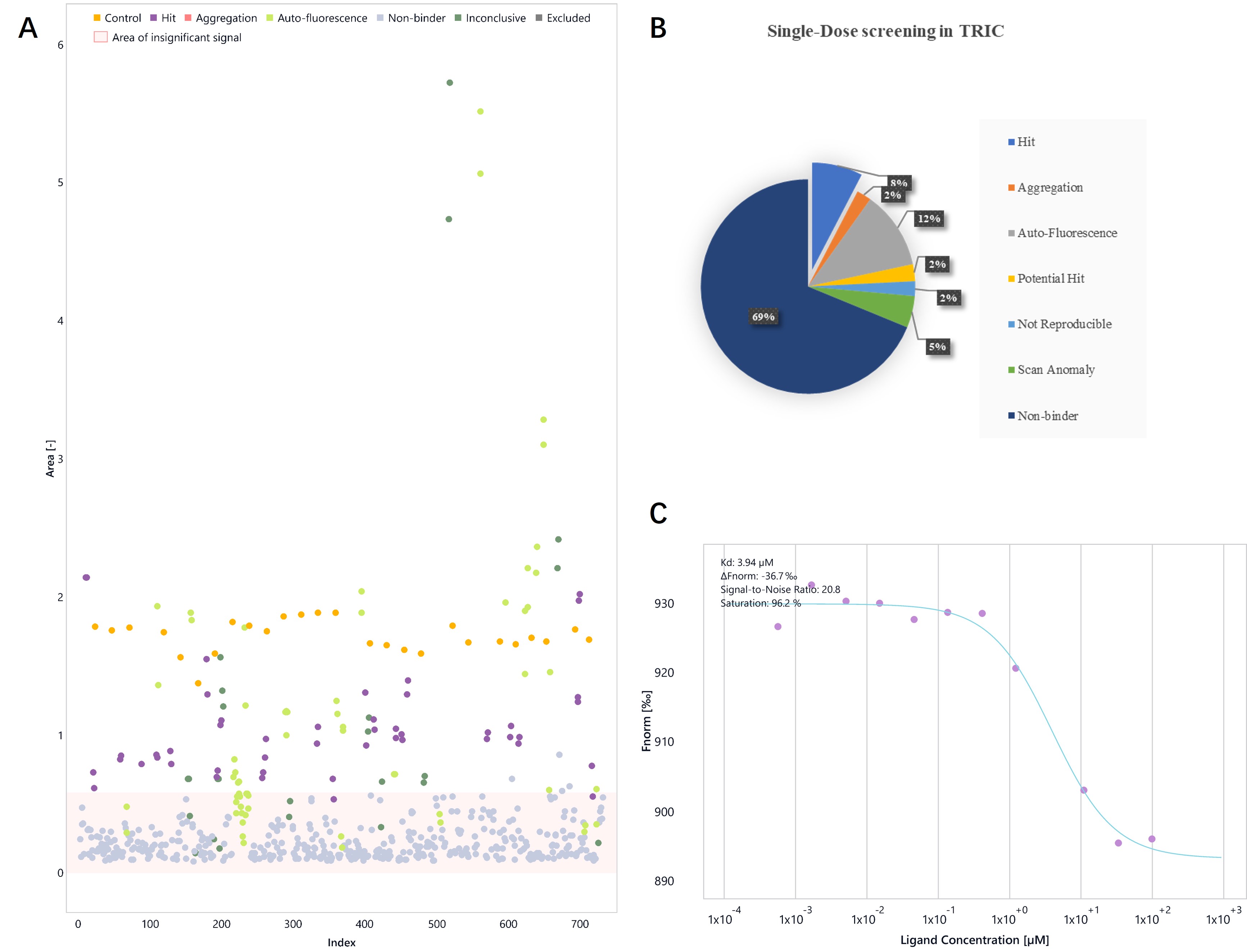
Figure. MST-TRIC supports BRD2 (BD2) drug discovery. A. Single-dose screening in MST-TRIC. Hits are shown in light purple. B. The pie chart summarizes the proportion of binders after the primary screen, which is 8%. C. The MST-TRIC binding affinity results indicate a hit to BRD2 (BD2).
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.