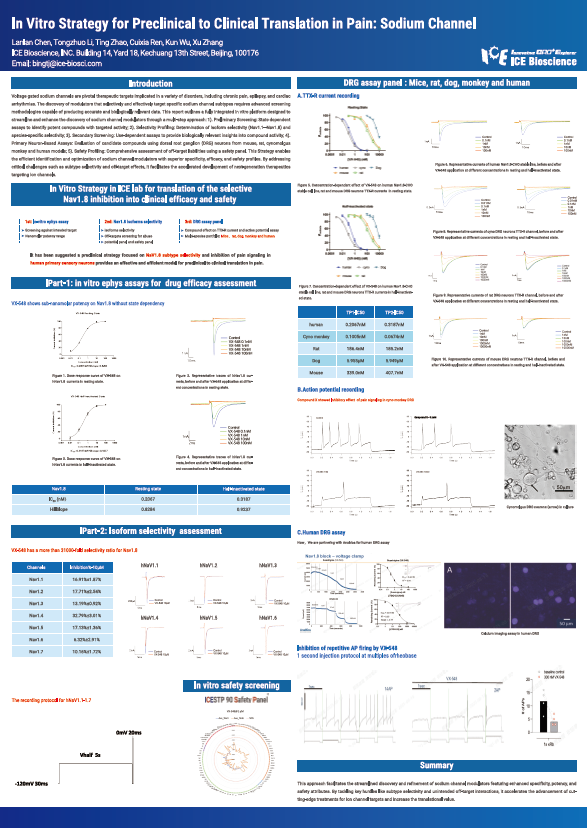
Voltage gated sodium channels are pivotal therapeutic targets implicated in a variety of disorders, including chronic pain, epilepsy, and cardiac arrhythmias. The discovery of modulators that selectively and effectively target specific sodium channel subtypes requires advanced screening methodologies capable of producing accurate and biologically relevant data. This report outlines a fully integrated in vitro platform designed to streamline and enhance the discovery of sodium channel modulators through a multi-step approach: 1). Preliminary Screening: State dependent assays to identify potent compounds with targeted activity; 2). Selectivity Profiling: Determination of isoform selectivity (Nav1.1–Nav1.8) and species-specific selectivity; 3). Secondary Screening: Use-dependent assays to provide biologically relevant insights into compound activity; 4). Primary Neuron-Based Assays: Evaluation of candidate compounds using dorsal root ganglion (DRG) neurons from mouse, rat, cynomolgus monkey and human models; 5). Safety Profiling: Comprehensive assessment of off-target liabilities using a safety panel. This Strategy enables the efficient identification and optimization of sodium channel modulators with superior specificity, efficacy, and safety profiles. By addressing critical challenges such as subtype selectivity and off-target effects, it facilitates the accelerated development of next-generation therapeutics targeting ion channels.

Antibody-drug conjugates (ADCs), combining the precision of antibodies with the potency of cytotoxic drugs, represent a promising anticancer therapy. However, current ADCs face significant challenges, including non-responsive cancers and rapid patient relapse, primarily due to tumor heterogeneity and drug resistance. To address these issues, dual-payload ADCs have emerged as a novel strategy, delivering two cytotoxic agents to enhance efficacy through synergistic effects, mitigate resistance, and offer flexible dosing. Despite their potential, the development of dual-payload ADCs remains complex. Cell panel-based studies and drug-resistant cell lines provide powerful tools to explore effective dual-payload ADCs by evaluating drug combination synergies and elucidating resistance mechanisms.

Obesity is a significant chronic disease that currently impacts approximately 40% of the global population, with projections from the World Obesity Atlas (WOA)1 indicating this figure could exceed 50% by 2035. Like other chronic conditions, obesity necessitates ongoing, lifelong management. Historically, long-term treatment out-comes for obesity have been disappointing and the challenges for obesity drug discovery include inefficient in vitro systems to evaluate multi-pathway interventions. Our comprehensive platform accelerates GPCR drug discovery by integrating High-throughput screening (HTS) in cell-based assay (reporter assay, HTRF cAMP, β-recruitment), supporting hit finding, balanced or biased compound (small molecular, peptides, antibody) test.

Targeted protein degradation (TPD) harnesses the ubiquitin-proteasome system to eliminate disease drivers once deemed “undruggable”. PROTACs recruit E3 ligases to targets via two linked warheads, while monovalent molecular glue degraders (MGDs) induce novel ligase-substrate interactions and ternary complex formation. Preclinical success in targeting VAV1, STAT6, NEK7, IRAK4 etc. has demonstrated remarkable efficacy in rheumatoid arthritis, atopic dermatitis and other autoimmune models, positioning TPD as a transformative therapeutic modality.
ICE’s integrated platform accelerates TPD discovery through SpS-based high-throughput screening, targeted libraries and an end-to-end workflow from hit identification to in vivo validation. Our experience includes support for discovery of multiple MGDs and PROTACs—showcased here by discovery of a potential NEK7 MGD, and our integrated TPD platform including orthogonal biochemical and biophysics binding assays, comprehensive in vitro functional characterization, in depth MOA studies, in vivo model validation and safety evaluation. By delivering robust, actionable data at every stage, ICE empowers rapid advancement of next-generation autoimmune interventions.