
High-throughput screening of a cancer cell panel represents a pivotal instrument in the armamentarium of drug discovery and development. The sensitivity profiles of these cell lines serve as a cornerstone for the refinement of in vivo model systems and for the stratification of patient cohorts in clinical trials. Werner Syndrome RecQ Helicase (WRN), a member of the RecQ helicase family, is instrumental in the preservation of genomic integrity. Its multifaceted role encompasses DNA repair, replication, recombination, and telomere maintenance. WRN has garnered significant attention as a potential therapeutic nexus in oncology, particularly within the context of tumors exhibiting microsatellite instability (MSI). The synthetic lethality between WRN and MSI has been the subject of intensive research, demonstrating that WRN inhibition is selectively toxic to cancer cells harboring a high microsatellite instability (MSI-H) phenotype, which is a characteristic observed across a spectrum of malignancies, including colorectal, endometrial, and gastric cancers.
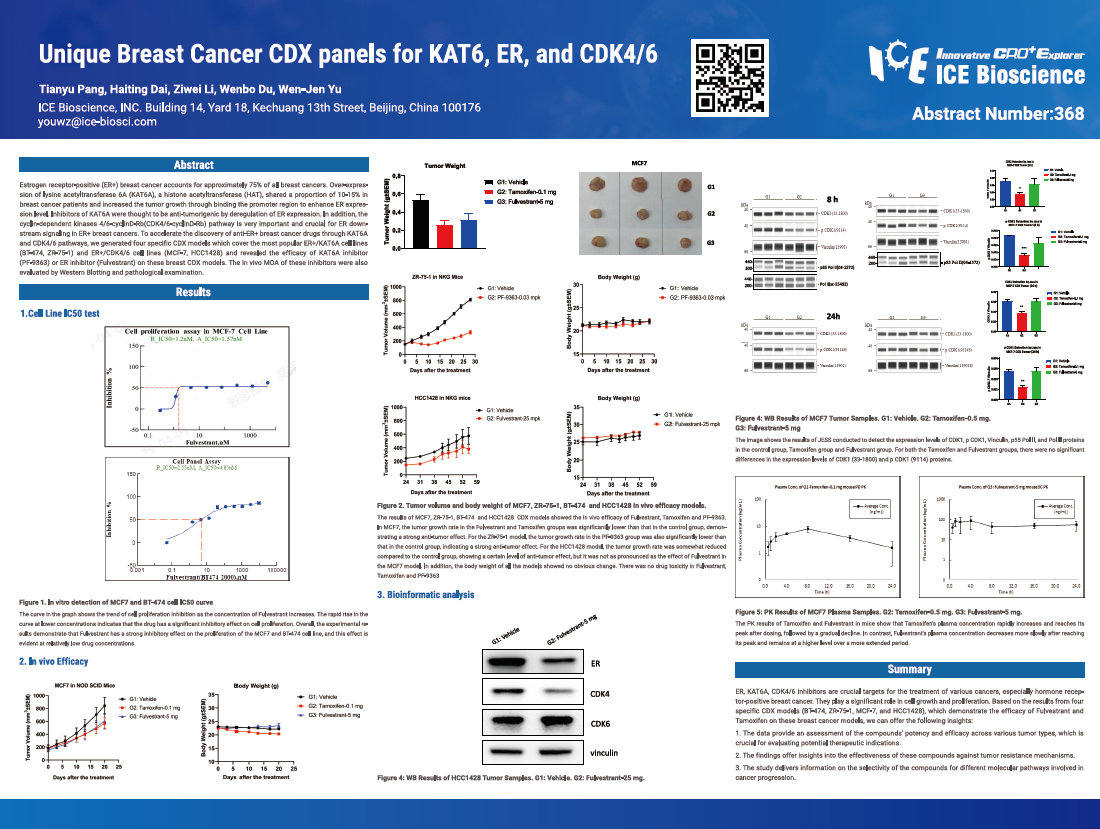
Estrogen receptor-positive (ER+) breast cancer accounts for approximately 75% of all breast cancers. Over-expression of lysine acetyltransferase 6A (KAT6A), a histone acetyltransferase (HAT), shared a proportion of 10-15% in breast cancer patients and increased the tumor growth through binding the promoter region to enhance ER expression level. Inhibitors of KAT6A were thought to be anti-tumorigenic by deregulation of ER expression. In addition, the cyclin-dependent kinases 4/6-cyclinD-Rb (CDK4/6-cyclinD-Rb) pathway is very important and crucial for ER downstream signaling in ER+ breast cancers. To accelerate the discovery of anti-ER+ breast cancer drugs through KAT6A and CDK4/6 pathways, we generated four specific CDX models which cover the most popular ER+/KAT6A cell lines (BT-474, ZR-75-1) and ER+/CDK4/6 cell lines (MCF-7, HCC1428) and revealed the efficacy of KAT6A inhibitor (PF-9363) or ER inhibitor (Fulvestrant) on these breast CDX models. The in vivo MOA of these inhibitors were also evaluated by Western Blotting and pathological examination.
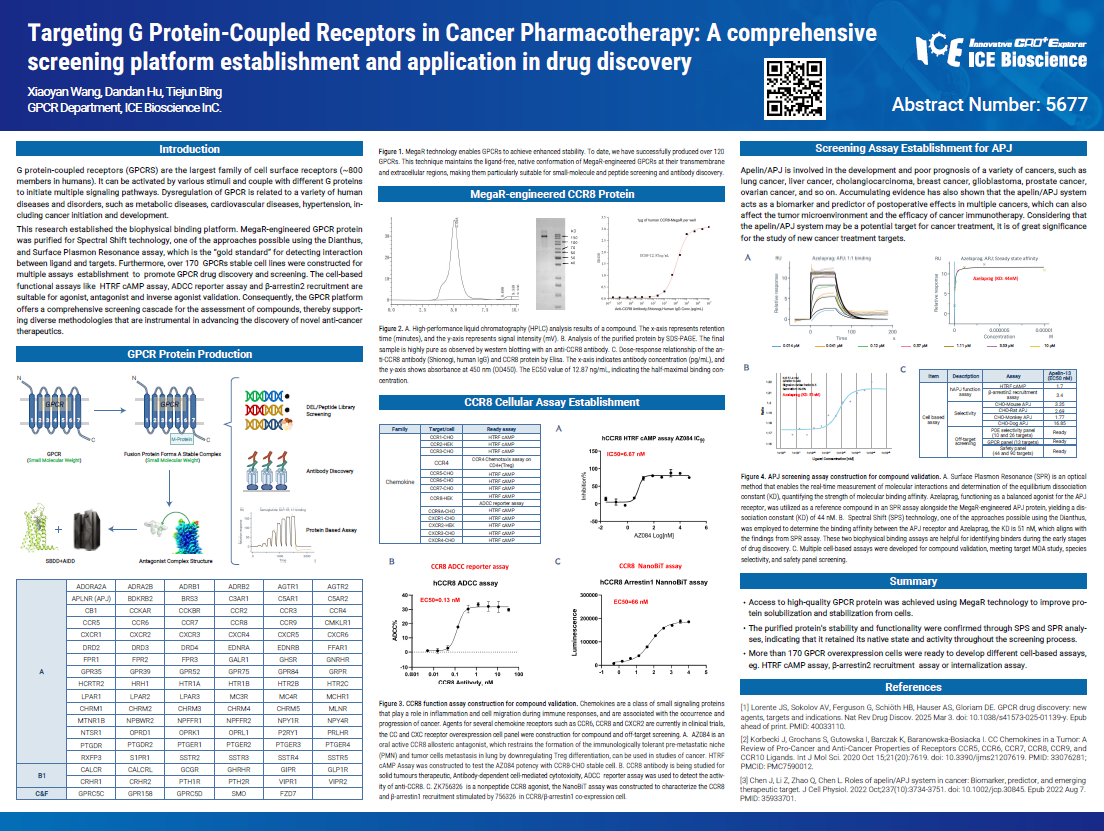
G protein-coupled receptors (GPCRS) are the largest family of cell surface receptors (~800 members in humans). It can be activated by various stimuli and couple with different G proteins to initiate multiple signaling pathways. Dysregulation of GPCR is related to a variety of human diseases and disorders, such as metabolic diseases, cardiovascular diseases, hypertension, including cancer initiation and development.
This research established the biophysical binding platform. MegaR-engineered GPCR protein was purified for Spectral Shift technology, one of the approaches possible using the Dianthus, and Surface Plasmon Resonance assay, which is the "gold standard" for detecting interaction between ligand and targets. Furthermore, over 170 GPCRs stable cell lines were constructed for multiple assays establishment to promote GPCR drug discovery and screening. The cell-based functional assays like HTRF cAMP assay, ADCC reporter assay and β-arrestin2 recruitment are suitable for agonist, antagonist and inverse agonist validation. Consequently, the GPCR platform offers a comprehensive screening cascade for the assessment of compounds, thereby supporting diverse methodologies that are instrumental in advancing the discovery of novel anti-cancer therapeutics.
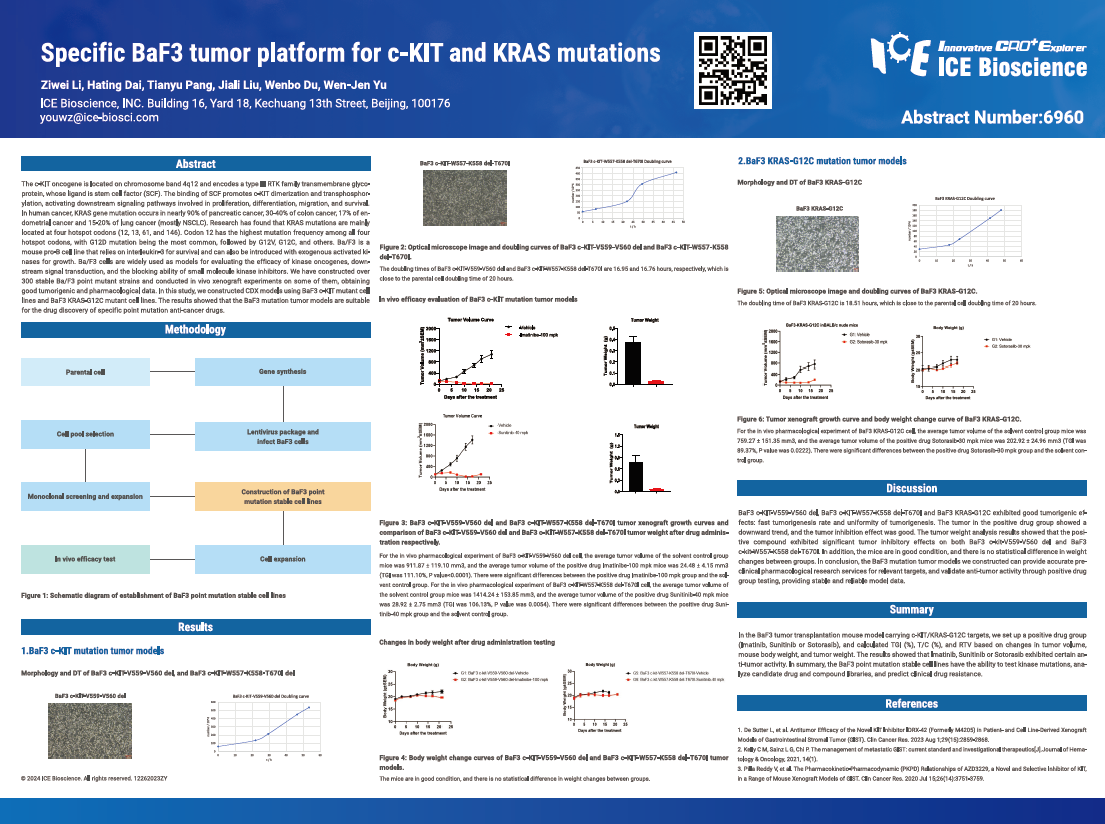
The c-KIT oncogene is located on chromosome band 4q12 and encodes a type Ill RTK family transmembrane glycoprotein, whose ligand is stem cell factor (SCF). The binding of SCF promotes c-KIT dimerization and transphosphorylation, activating downstream signaling pathways involved in proliferation, differentiation, migration, and survival. In human cancer, KRAS gene mutation occurs in nearly 90% of pancreatic cancer, 30-40% of colon cancer, 17% of endometrial cancer and 15-20% of lung cancer (mostly NSCLC). Research has found that KRAS mutations are mainly located at four hotspot codons (12, 13, 61, and 146). Codon 12 has the highest mutation frequency among all four hotspot codons, with G12D mutation being the most common, followed by G12V, G12C, and others. Ba/F3 is a mouse pro-8 cell line that relies on interleukin-3 for survival and can also be introduced with exogenous activated kinases for growth. Ba/F3 cells are widely used as models for evaluating the efficacy of kinase oncogenes, downstream signal transduction, and the blocking ability of small molecule kinase inhibitors. We have constructed over 300 stable Ba/F3 point mutant strains and conducted in vivo xenograft experiments on some of them, obtaining good tumorigenic and pharmacological data. In this study, we constructed CDX models using BaF3 c-KIT mutant cell lines and BaF3 KRAS-G12C mutant cell lines. The results showed that the BaF3 mutation tumor models are suitable for the drug discovery of specific point mutation anti-cancer drugs.