ICE Bioscience, your trusted partner in advancing pharmaceutical innovation through cutting-edge in vivo pharmacokinetics (PK) services. Our comprehensive suite of services covers a spectrum of offerings in the drug development process, ensuring a thorough evaluation of candidate compounds at the whole-body level.
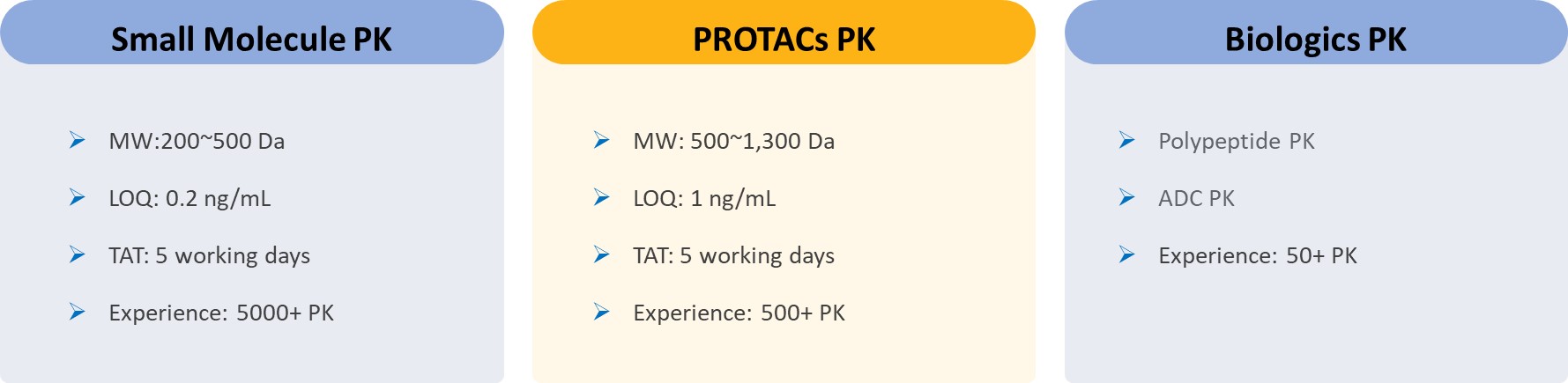
Conventional PK Study Service Offerings:
✅ Species: Mouse/Rat/Dog/Monkey
✅ Administration Methods: IV/PO/SC/IP/IM
✅ Dosage Forms: Single/Multiple/Cassette Dosing
✅ PK Models: Full PK/Rapid PK/Snapshot PK/Cassette PK
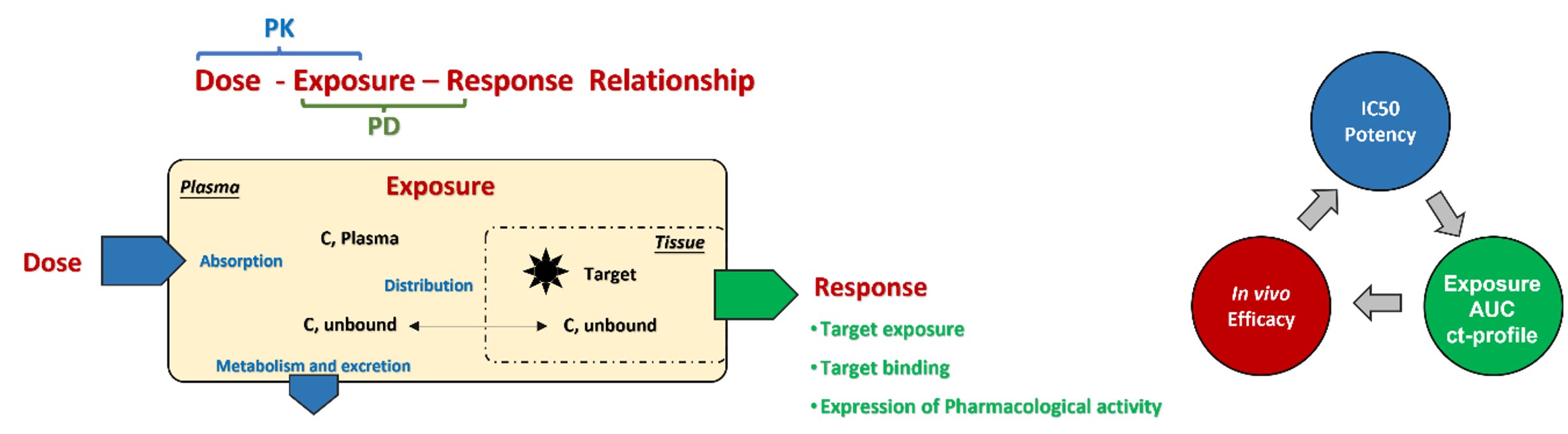
PK-Related Services:
✅ Tissue Distribution/Blood-Brain Barrier
✅ Excretion/Renal Clearance
✅ Quantitative Detection of Metabolites
✅ Formulation Pretest
In Vivo BDC PK:
✅ Biliary Duct Intubation Surgery
✅ Metabolic Cage
✅ Bilirubin Determination
✅ Intravenous Infusion
✅ Collection of Bile, Urine, and Feces
✅ Metabolite Identification
✅ Cumulative Excretion Rate
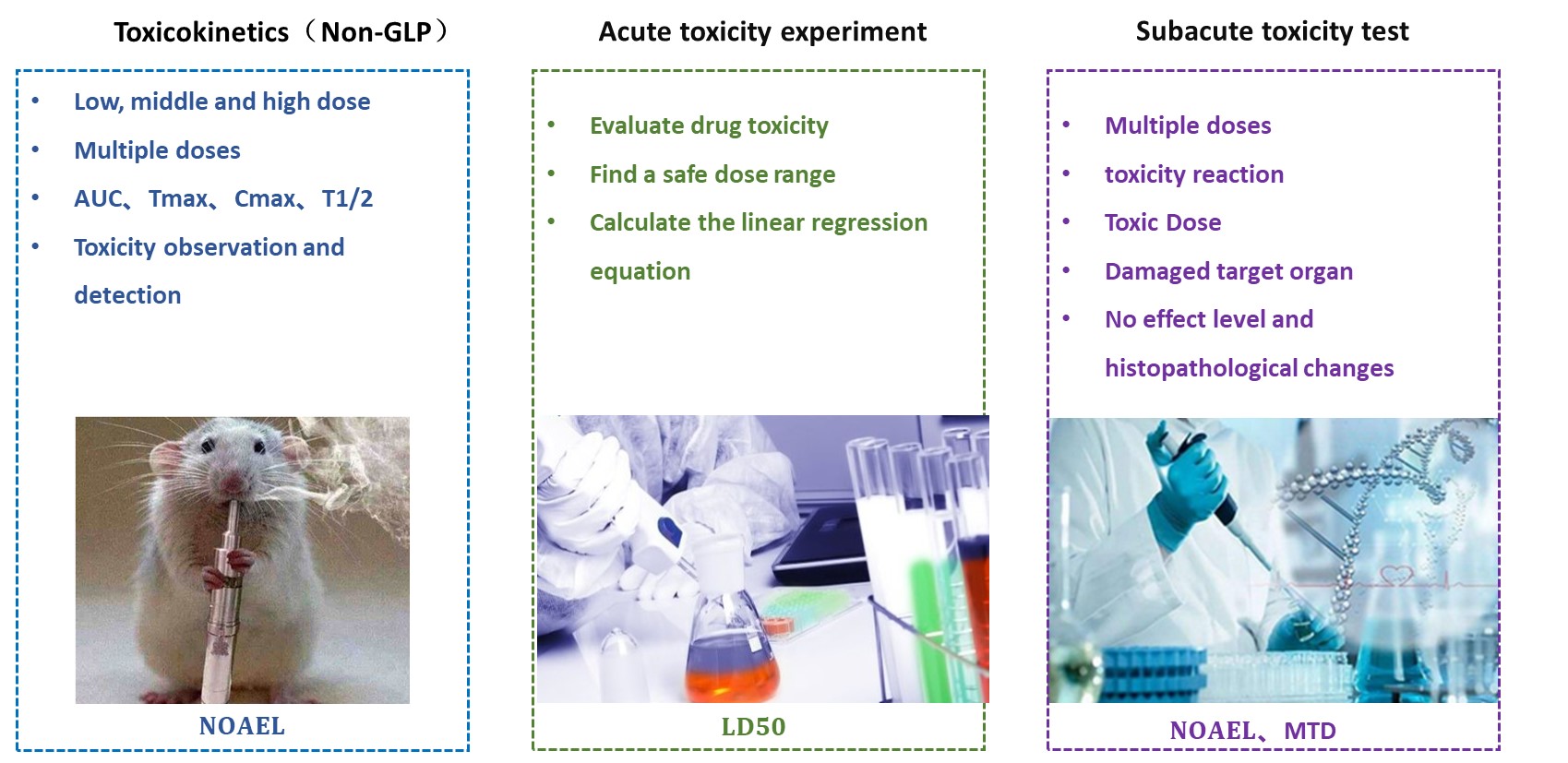
PK-Toxicity Evaluation Assays:
✅ Evaluate Drug Toxicity
✅ Find a Safe Dose Range
✅ Estimate LD50
✅ Calculate the Linear Regression Equation
✅ Toxicity Observation
✅ Biochemical and Pathological Analysis
CNS-In Vivo PK:
✅ Cerebrospinal Fluid
✅ Brain Homogenate
✅ Unbound Level in Brain
✅ Dorsal Raphe Nucleus
✅ Hippocampus
✅ Corpus Striatum
✅ Cerebral Cortex
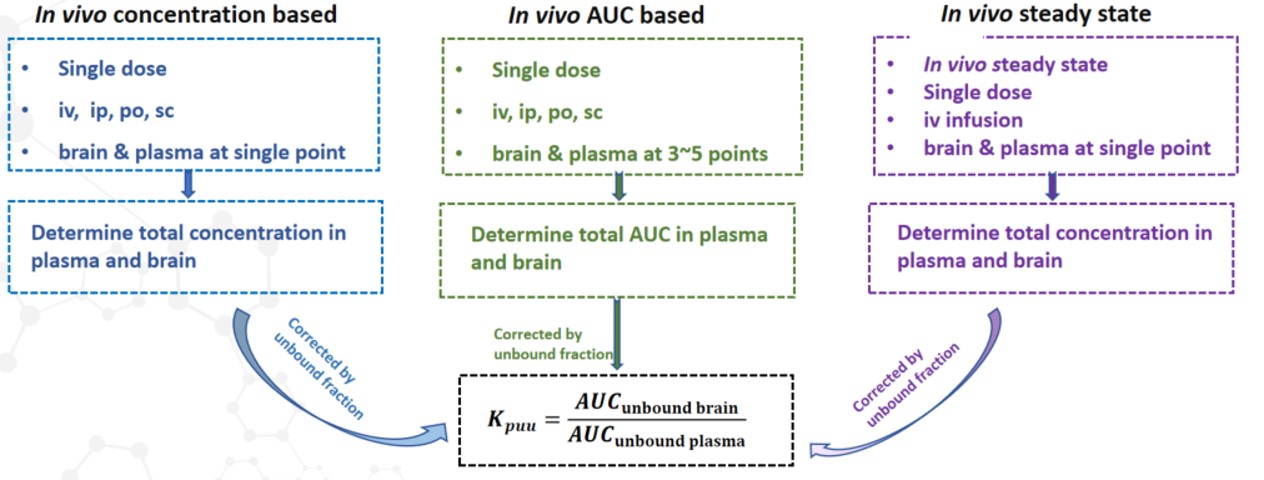
✅ Kpuu
✅ Kp
In summary, in vivo PK services contribute invaluable data to drug development by elucidating how drugs interact with living systems. These studies are essential for making informed decisions about drug candidates, optimizing formulations, and assessing potential toxicities. The information obtained through in vivo PK studies is critical for advancing drug development pipelines and ultimately bringing safe and efficacious medicines to the market.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.
Address: Bldg 16, Yd 18, Kechuang 13th St, Etown, Tongzhou Dist, Beijing, 100176, China
Email: marketing@ice-biosci.com
Tel:+86-10-67809840

