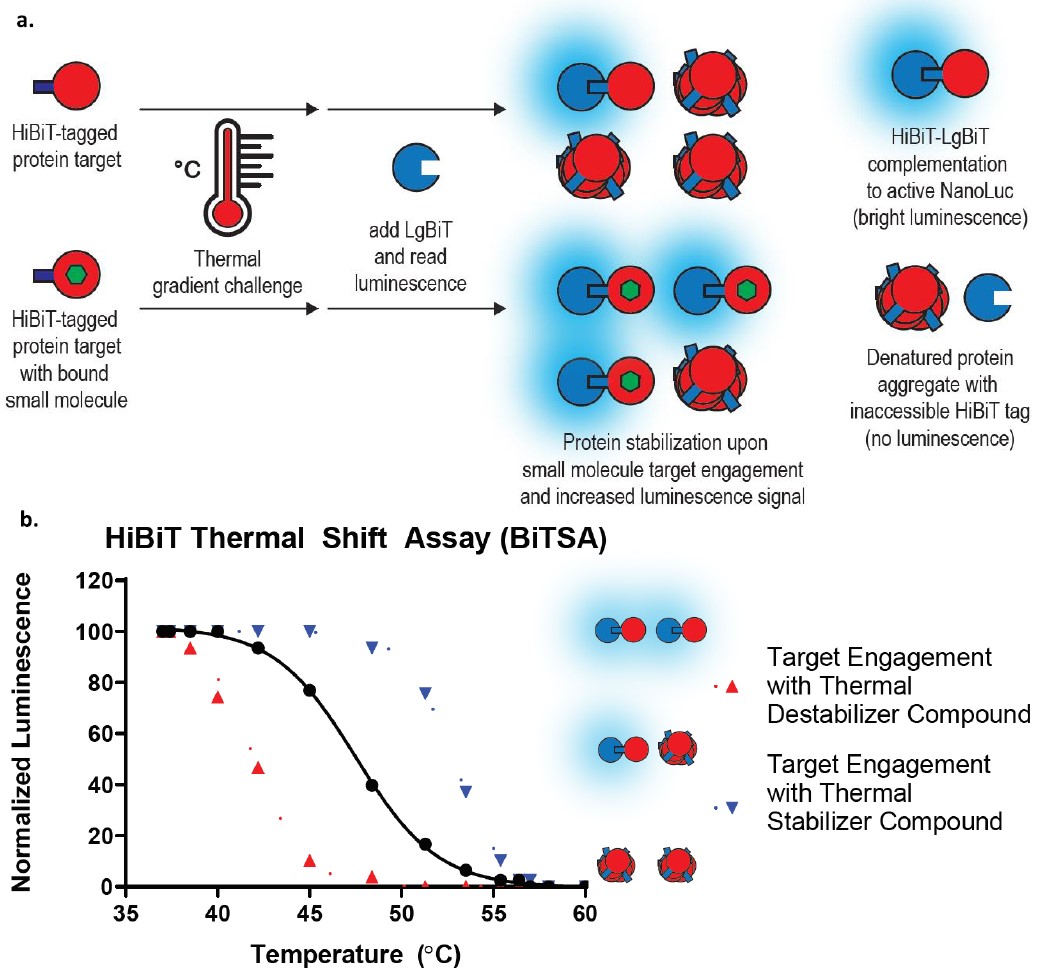
BiTSA (Bioluminescent Thermal Shift Assay) is an advanced technique adapted from CETSA (Cellular Thermal Shift Assay) for evaluating drug-target interactions within cells. CETSA measures changes in protein thermal stability upon ligand binding, indicating direct target engagement. BiTSA enhances this method with a bioluminescent readout, enabling rapid and high-throughput screening.
BiTSA Procedure and Principle
Cells expressing HiBiT-tagged target proteins are treated with test compounds and subjected to a thermal gradient to induce protein denaturation. The HiBiT tag, a small peptide, binds to its complementary partner LgBiT to form a luminescent enzyme. During thermal challenge, proteins that remain soluble retain the HiBiT tag, which can then bind to LgBiT and produce a luminescent signal. The intensity of the luminescent signal correlates with the amount of stabilized, soluble protein, providing a quantitative measure of target engagement[1].
ACS Med Chem Lett . 2021 Jul 12;12(8):1288-1294.
Why BiTSA is Needed in Drug Discovery
Target Engagement Measurement: BiTSA allows for the direct assessment of drug binding to target proteins within their native cellular environment, which is crucial for confirming the mechanism of action and efficacy of drug candidates.
Higher Sensitivity and Quantitative Measurement: BiTSA uses a bioluminescent readout, which is more sensitive than the traditional methods used in CETSA, and simplifies the assay procedure, reducing the time and effort required for sample preparation.
High-Throughput Screening: The luminescent readout of BiTSA allows for rapid, high-throughput assessment of drug-target interactions, facilitating efficient screening of large compound libraries.
✅ Fast Development: 5-7 days to develop the assay for new targets.
✅ High Throughput: Capable of screening large compound libraries using a 384-well plate format.
✅ Quick Turnaround: Results can be obtained within 2 days, enabling rapid progression through the drug discovery pipeline.
✅ Automation: Automatable, allowing for high-throughput screening and reducing manual intervention.
p38α, also known as MAPK14, is a member of the p38 MAP kinase family. It plays a crucial role in cellular responses to stress and inflammation. The stabilization of p38α upon ligand binding can be used as a measure of drug-target engagement. The original CETSA method showed significant stabilization of p38α in HL-60 cells, providing a benchmark for evaluating our BiTSA results.
Literature Benchmark
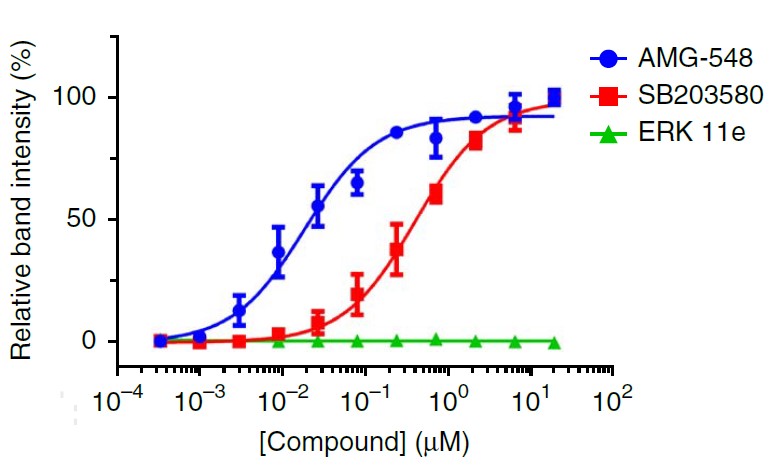
The original CETSA method showed significant stabilization of p38α in HL-60 cells, with an EC50 of 35±16 nM[2].
ICE Bioscience Data
DATA 1: Temperature-Dependent Stabilization
Objective: Determine the temperature at which MAPK14-HiBiT remains stabilized in the presence of AMG 548.
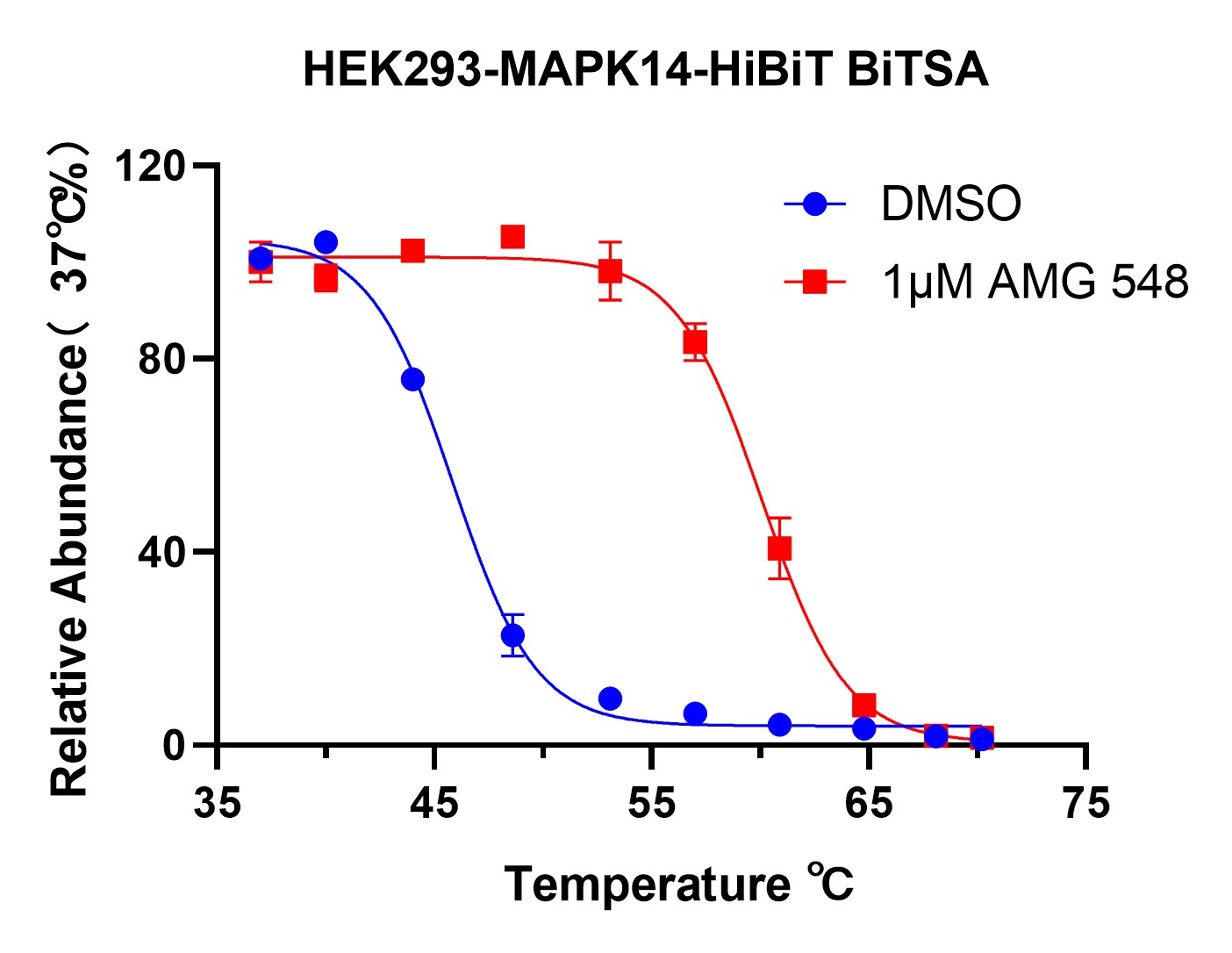
Results: HEK293-MAPK14-HiBiT cells were treated with DMSO or 1μM AMG 548 and subjected to a temperature gradient. The relative abundance of stabilized protein was plotted against temperature, showing significant stabilization at 54°C in the presence of AMG 548 compared to the DMSO control.
DATA 2: Dose-Dependent Stabilization
Objective: Determine the dose-response relationship of AMG 548 on MAPK14-HiBiT stabilization at a fixed temperature (54°C).
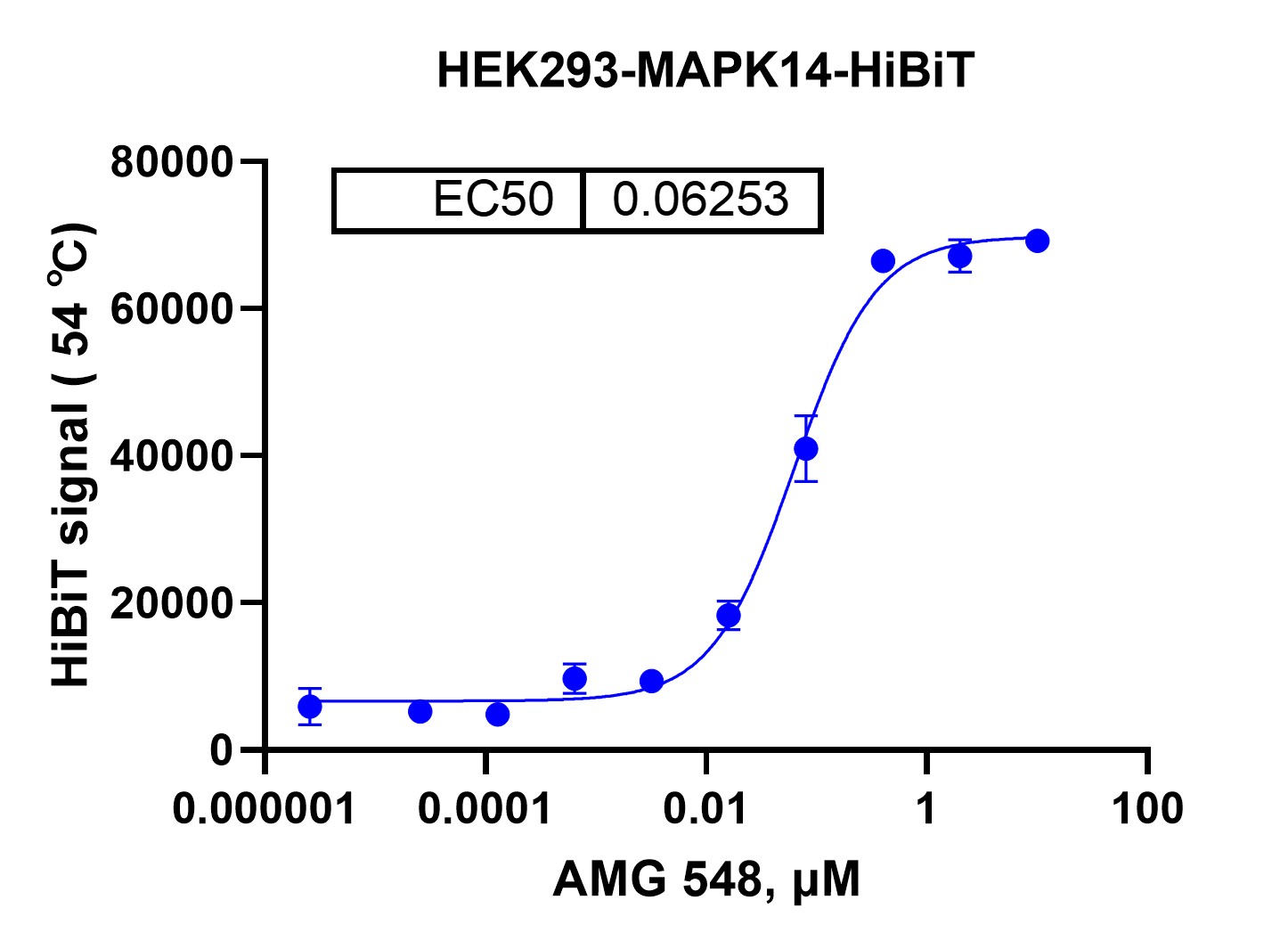
Results: HEK293-MAPK14-HiBiT cells were treated with varying concentrations of AMG 548 (0.000001 to 100 μM). The HiBiT signal was measured, revealing a EC50 (dose required for half-maximal thermal stabilization) of 0.06253 μM.
Future Directions
ICE Bioscience plans to extend the application of BiTSA to study membrane proteins, leveraging the same principles of thermal stabilization to explore drug interactions with more complex targets.
ICE Bioscience has effectively established BiTSA, with results that are consistent with established literature, demonstrating our capability in advanced drug discovery assays. This positions us well for future explorations into more complex protein targets, expanding our contributions to the field of biopharmaceuticals.
References
[1] Jonathan D. Mortison, Ivan Cornella-Taracido, Gireedhar Venkatchalam, Anthony W. Partridge, Nirodhini Siriwardana, & Simon M. Bushell. (2021). Rapid Evaluation of Small Molecule Cellular Target Engagement with a Luminescent Thermal Shift Assay. ACS Med. Chem. Lett., 12, 1288-1294.
[2] Daniel M. Molina, Ramin Jafari, Helena Ignatushchenko, Martin Seki, Ekaterina A. Larsson, Christoph Dan, Lalit Sreekumar, Yan He Cao, & Pär Nordlund. (2014). The cellular thermal shift assay for evaluating drug target interactions in cells. Nature Protocols, 9(9), 2100-2122.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.