PRMT5 (Protein Arginine Methyltransferase 5) is a crucial enzyme in the regulation of gene expression, RNA processing, and DNA repair. It achieves these functions by symmetrically dimethylating arginine residues on substrate proteins, a modification known as SDMA (Symmetric Dimethylarginine). This enzymatic activity is heavily implicated in the development and progression of various cancers, making PRMT5 an important target for therapeutic intervention.
PRMT5 does not act alone; it requires the cofactor MEP50 (Methylosome Protein 50, also known as WDR77) to fully exert its methyltransferase activity. The PRMT5/MEP50 complex is responsible for the efficient methylation of substrate proteins, which includes histones and other key regulatory proteins. The formation of this complex is essential for the enzymatic function of PRMT5, and its activity is tightly regulated by several factors, including the availability of cofactors like SAM (S-Adenosylmethionine). SAM is a universal methyl donor involved in numerous methylation reactions across the cell, including those catalyzed by PRMT5. SAM donates the methyl group that PRMT5 transfers to arginine residues, leading to the production of SDMA. The availability of SAM is crucial for the optimal activity of PRMT5. However, the regulatory landscape becomes more complex with the presence of MTA (Methylthioadenosine), a byproduct of polyamine biosynthesis that can accumulate in certain cancer cells due to MTAP (Methylthioadenosine Phosphorylase) gene deletions.
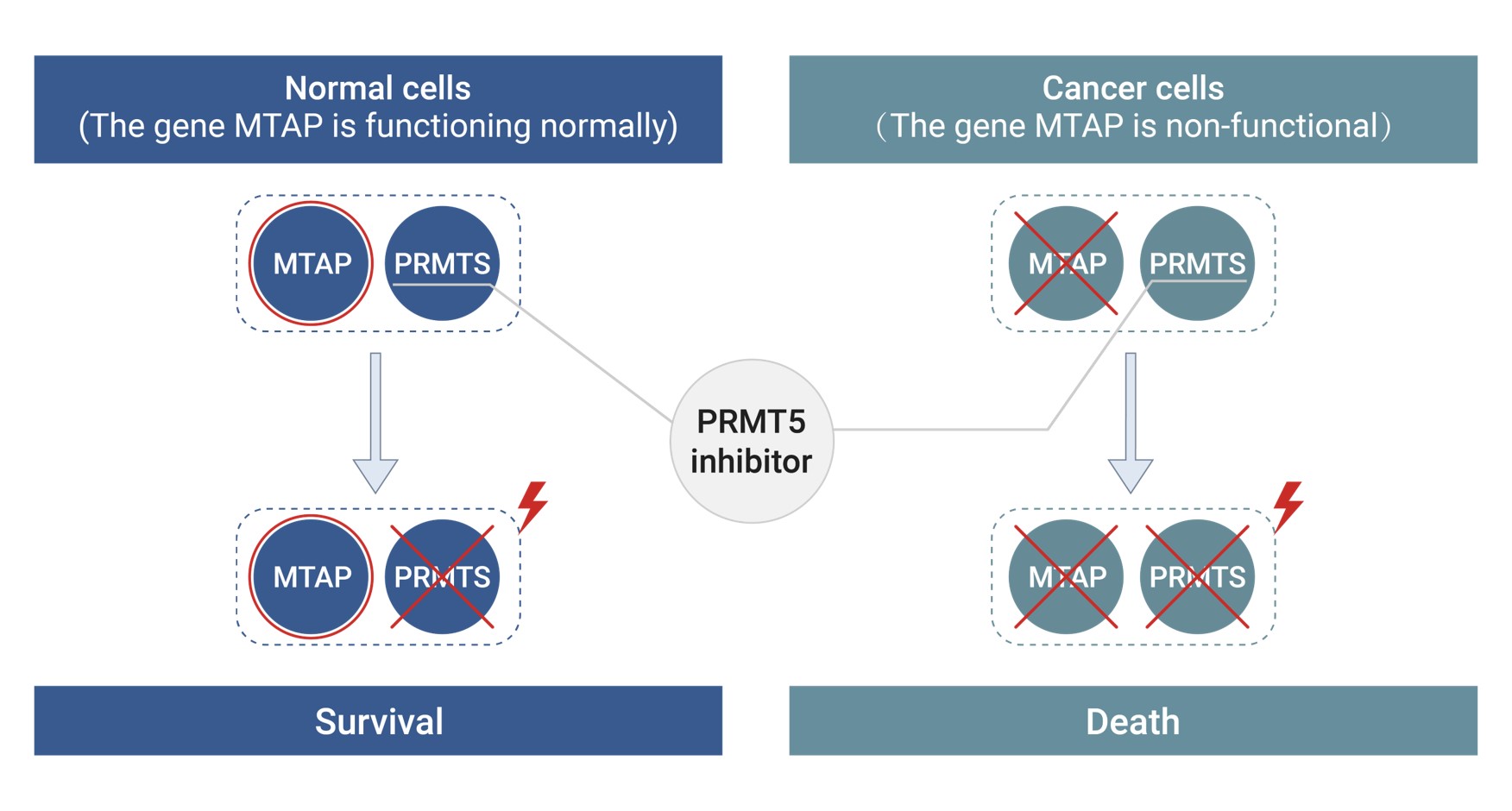
MTAP Deletion and Synthetic Lethality
MTAP deletion often co-occurs with CDKN2A loss in various cancers, such as pancreatic and non-small cell lung cancer. This deletion leads to the accumulation of MTA, a natural inhibitor of PRMT5. Cancer cells with MTAP deletions are highly dependent on PRMT5 for survival, making them susceptible to synthetic lethality when PRMT5 is inhibited. This unique vulnerability presents an opportunity to selectively target cancer cells while sparing normal cells, a strategy that is increasingly being explored with PRMT5 inhibitors.
Surface Plasmon Resonance (SPR) is a powerful tool used to study the binding interactions between PRMT5/MEP50 and potential inhibitors. In this context, SPR has been employed to analyze the binding affinities and kinetics of small-molecule inhibitors, such as MRTX1719.
Given that MTA and SAM (S-Adenosylmethionine) both play pivotal roles in regulating PRMT5 activity, a detailed SPR analysis was conducted to compare their binding to the PRMT5/MEP50 complex. MTA exhibited a significantly stronger binding affinity to the PRMT5/MEP50 complex than SAM. These findings are crucial for understanding the role of MTA in the inhibition of PRMT5, particularly in cancer cells with MTAP deletions, where MTA accumulates and competes with SAM. The higher affinity of MTA suggests that in MTAP-deleted cancers, where MTA levels are elevated, the inhibitory effect on PRMT5 could be more pronounced, potentially enhancing the effectiveness of PRMT5 inhibitors like MRTX1719.
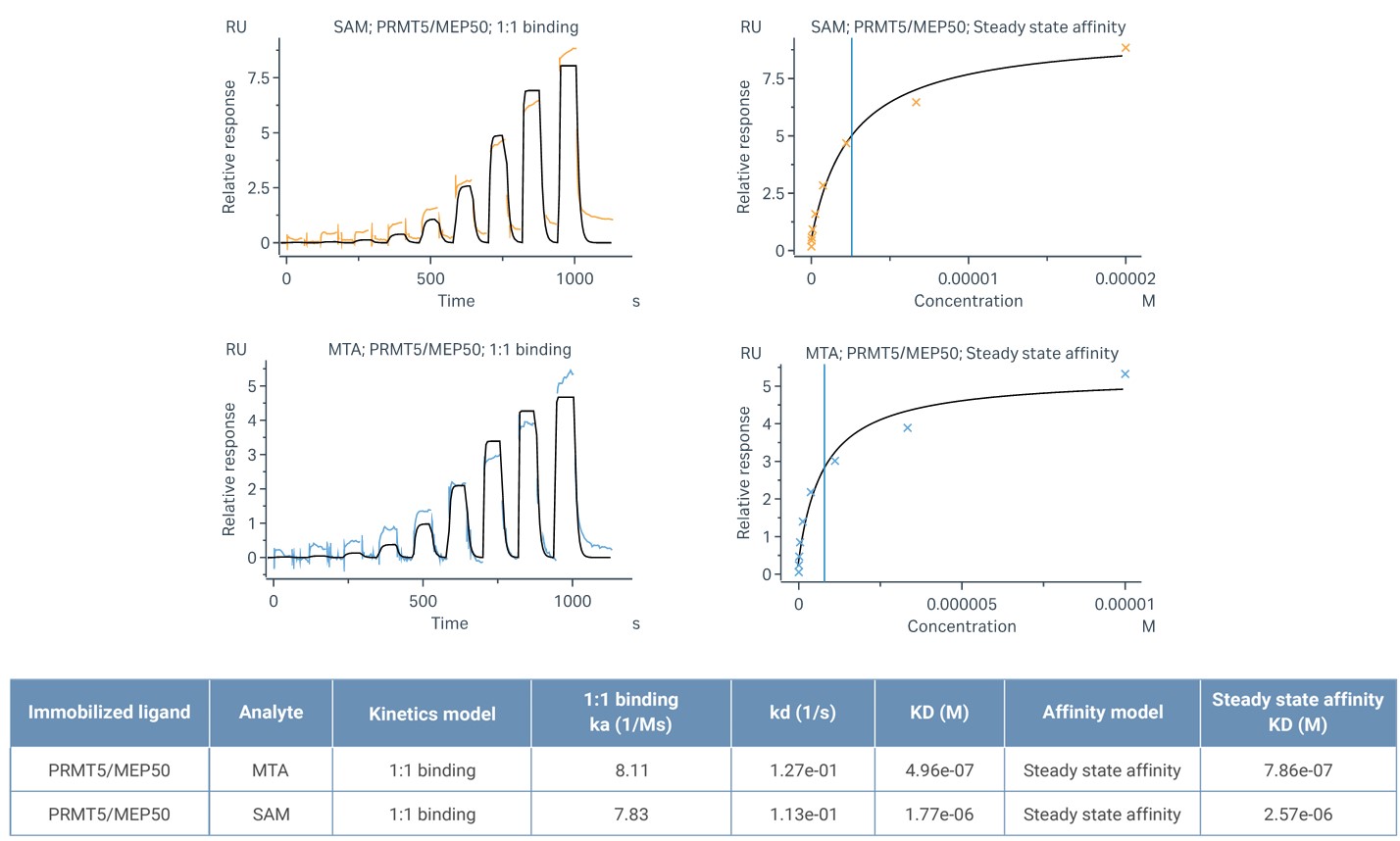
Figure 1. SPR analysis demonstrated the binding affinity of SAM and MTA to the PRMT5/MEP50 complex.
In addition, the study examined MRTX1719 in both the presence and absence of MTA to understand its impact on the PRMT5/MEP50 complex. The presence of MTA can influence the binding dynamics, revealing how MTA might modulate the inhibitor’s efficacy. This analysis is crucial for identifying potential resistance mechanisms and optimizing therapeutic strategies.
Our SPR analysis demonstrated that MTA acts to stabilize the MRTX1719-PRMT5/MEP50 complex, making MRTX1719 more effective as an inhibitor when MTA is present. This is particularly relevant in the context of MTAP-deleted cancers, where MTA accumulates, potentially enhancing the therapeutic efficacy of MRTX1719.

Figure 2. SPR analysis of MRTX1719's binding affinity to the PRMT5/MEP50 complex under conditions with and without MTA.
Complementing the SPR studies, biochemical assays were conducted to assess the inhibitory effects of various compounds on PRMT5 activity. One of the key assays used was the MTase-Glo assay, a luminescence-based method that measures methyltransferase activity. The MTase-Glo assay is highly sensitive, allowing for precise quantification of enzyme inhibition across a broad range of inhibitor concentrations. In this study, the MTase-Glo assay was used to evaluate the inhibitory effects of GSK326595 and LLY-283 on PRMT5 activity.
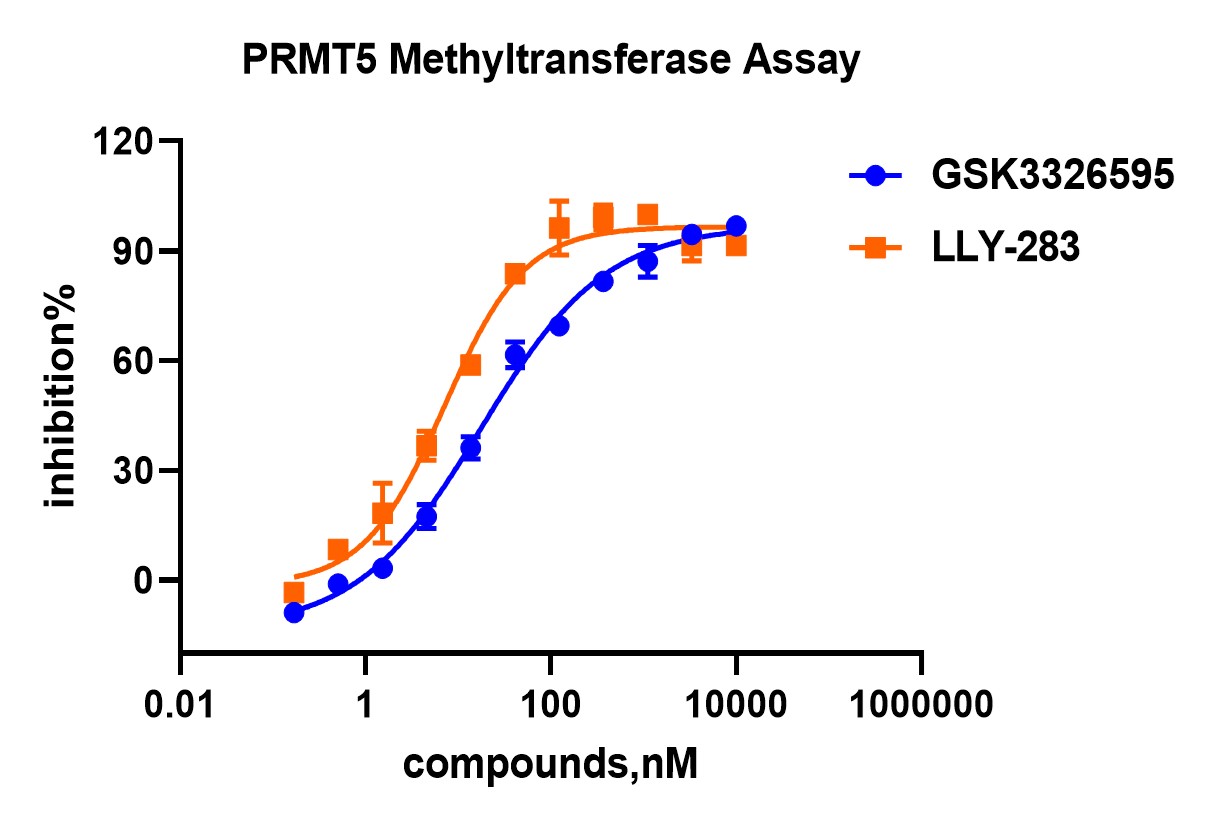
Figure 3. Assessment of the inhibitory effects of diverse compounds on PRMT5 activity.
SAM Competition: This result suggests that LLY-283 acts as a competitive inhibitor with respect to SAM. As SAM concentration increases, LLY-283 is less effective at inhibiting PRMT5, as evidenced by the increasing IC50.
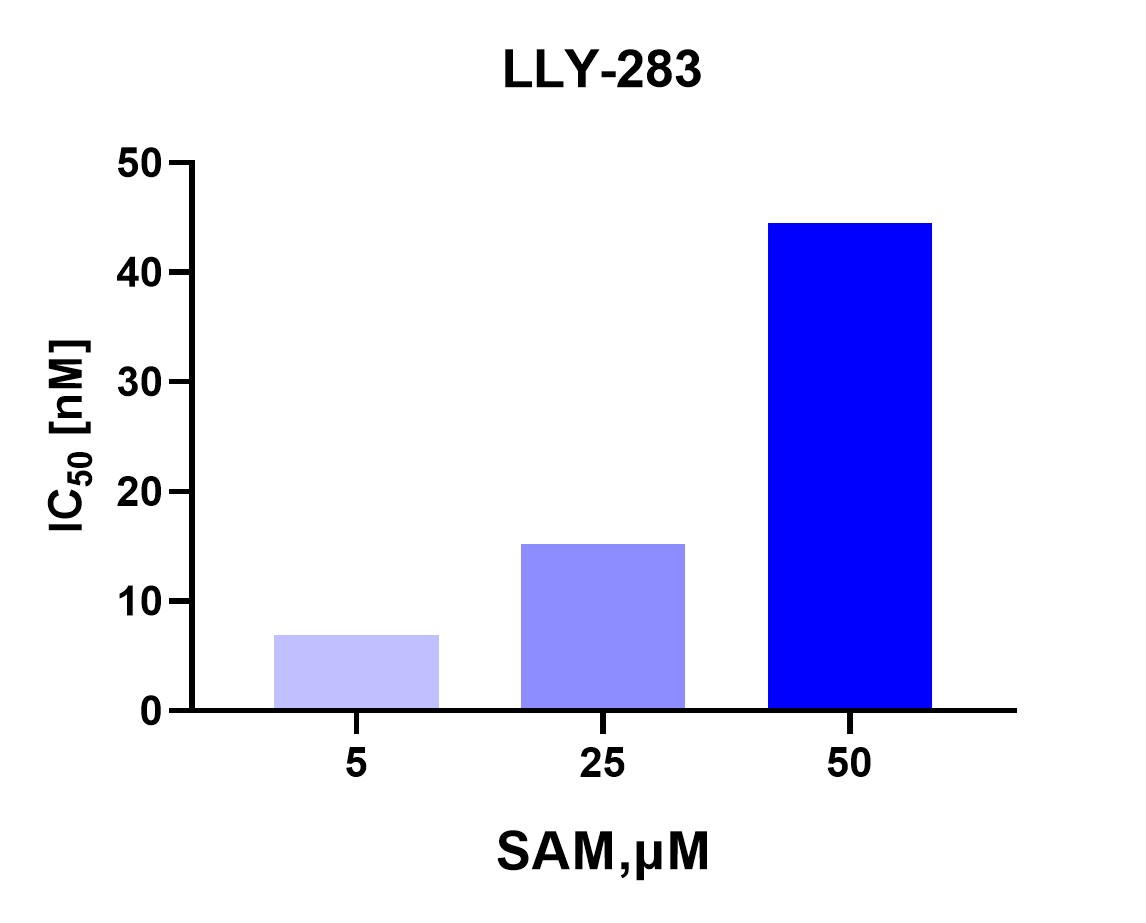
Figure 4. SAM-Competitive Test
For MRTX1719, the assays focused on measuring PRMT5/MEP50 activity both in the presence and absence of MTA. These biochemical assay results provide functional validation of the SPR conclusions: the presence of MTA significantly enhances the inhibitory potency of MRTX1719 against PRMT5/MEP50. Specifically, MTA not only increased the binding affinity observed in SPR but also led to a significant decrease in the IC50 of MRTX1719, meaning it requires a much lower concentration to achieve the same level of inhibition in the presence of MTA.
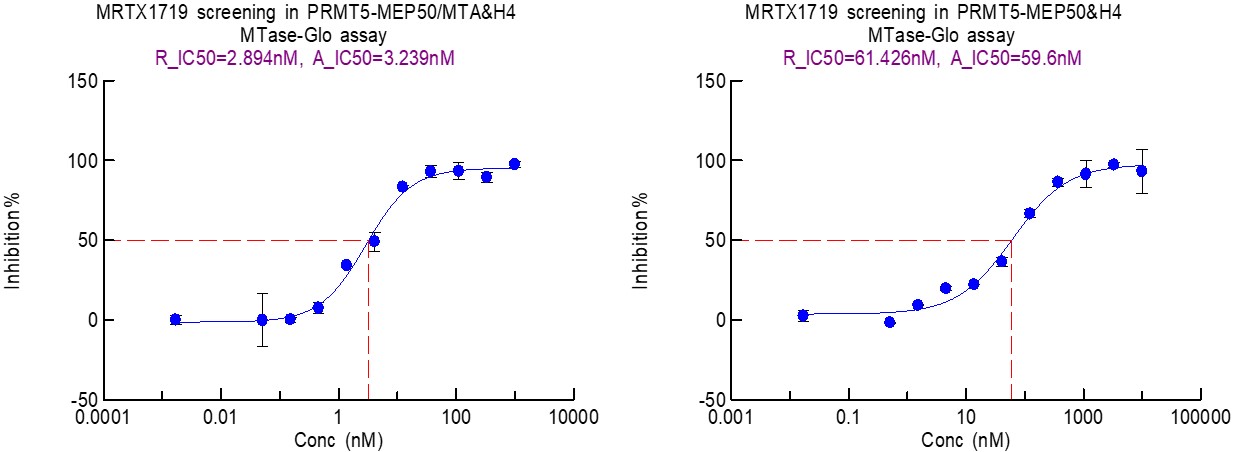
Figure 5.Measurement of the inhibitory effect of the MTA-Cooperative PRMT5 inhibitor MRTX1719 on PRMT5 activity in the presence and absence of MTA.
Moreover, a broad specificity analysis was conducted across several PRMT family members, including PRMT1, PRMT3, PRMT4, PRMT5, PRMT6, and PRMT8. Using a fixed compound concentration of 10 µM, it was found that only PRMT5’s activity was significantly inhibited, while the activities of other PRMTs remained largely unaffected. This selectivity highlights the potential of these inhibitors to specifically target PRMT5 without off-target effects on other PRMT enzymes, underscoring their therapeutic value in cancers where PRMT5 activity needs to be precisely modulated.
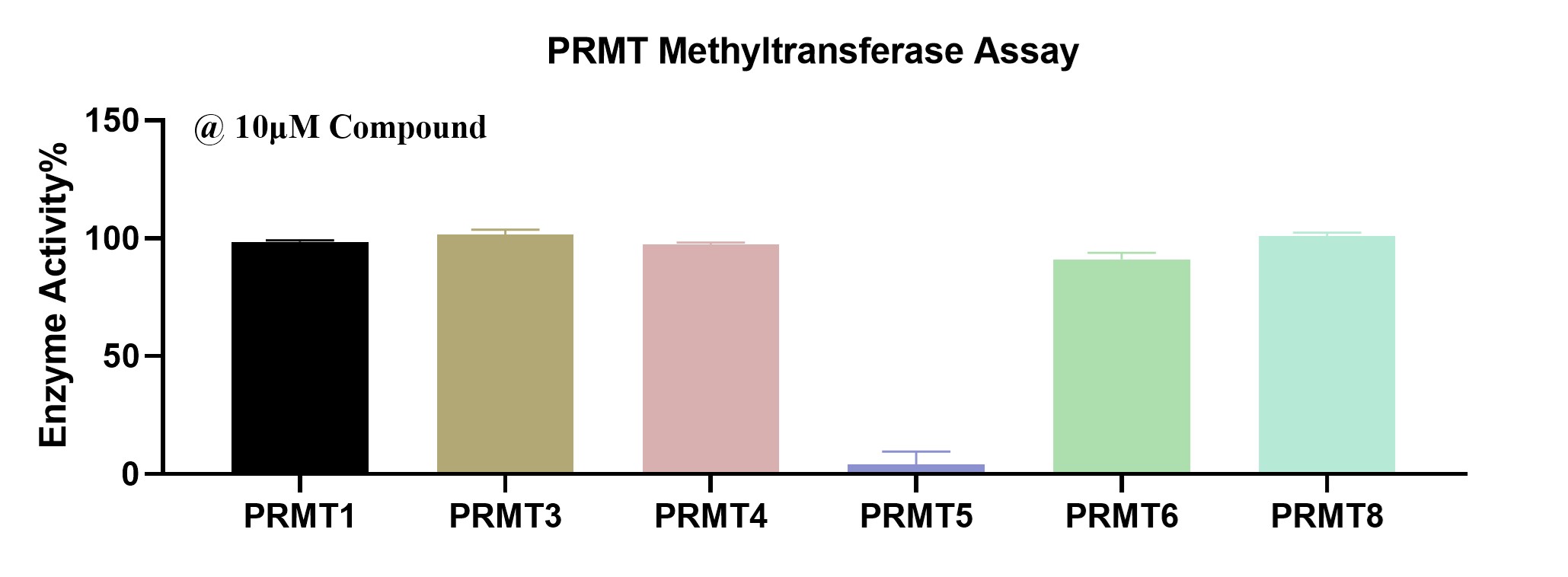
Figure 6. PRMT Methyltransferase Panel Screen
Using cell proliferation and in-cell western assays, we evaluated the effects of the PRMT5 inhibitor MRTX1719 on cell proliferation and SDMA levels in both HCT116 WT and MTAP KO cells. The results showed significant inhibition of cell growth and alterations in SDMA levels, highlighting MRTX1719’s potential as a therapeutic agent. PRMT5 is known to symmetrically dimethylate arginine residues on target proteins, and SDMA is a marker of this activity. The observed changes in SDMA levels upon treatment with MRTX1719 further confirm its role in inhibiting PRMT5. Further research will explore its mechanism of action and safety profile.
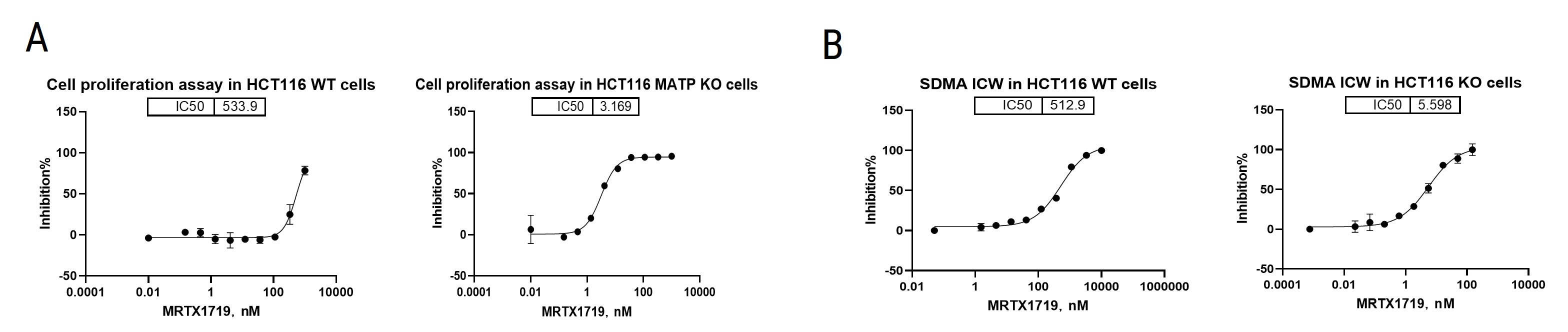
Figure 7. (A) Cell proliferation assay in HCT116 WT and MTAP KO cells. (B) Measurement of SDMA in HCT116 WT and MTAP KO cells by in-cell western assays (ICW).
The degradation of PRMT5 by PROTAC could be analyzed using HiBiT knock-in cell lines or Jess assays. The HCT116-PRMT5-HiBiT cell line was verified by siRNA. Jess assay analysis in HCT116 cells showed that PROTAC induced degradation of PRMT5. Our findings highlight the potential of PROTACs in targeted protein degradation.
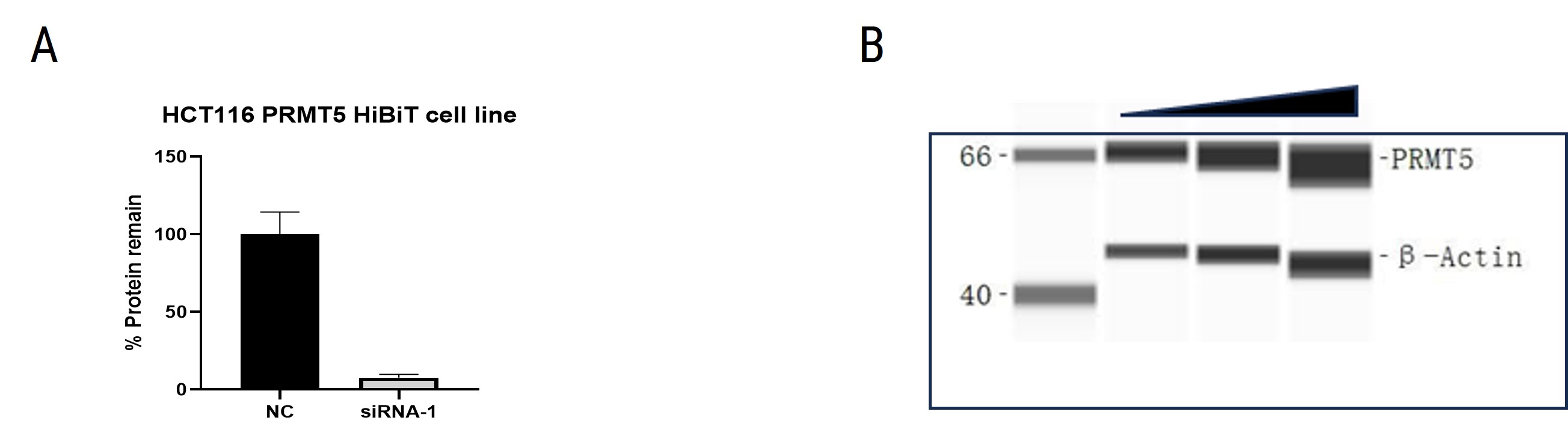
Figure 8. (A) Verification of HCT116-PRMT5-HiBiT cell line by siRNA. (B) Jess assay analysis of PRMT5 in HCT116 cells.
The in vivo efficacy of MRTX1719 was measured using the HCT116-KO-MTAP CDX model. Our investigation focused on assessing the effects of MRTX1719 treatment on body weight, tumor volume, and tumor weight in the CDX model. The results demonstrated a significant reduction in both tumor volume and tumor weight following MRTX1719 treatment, indicating its potential as an effective therapeutic agent for cancers with MTAP deletions.
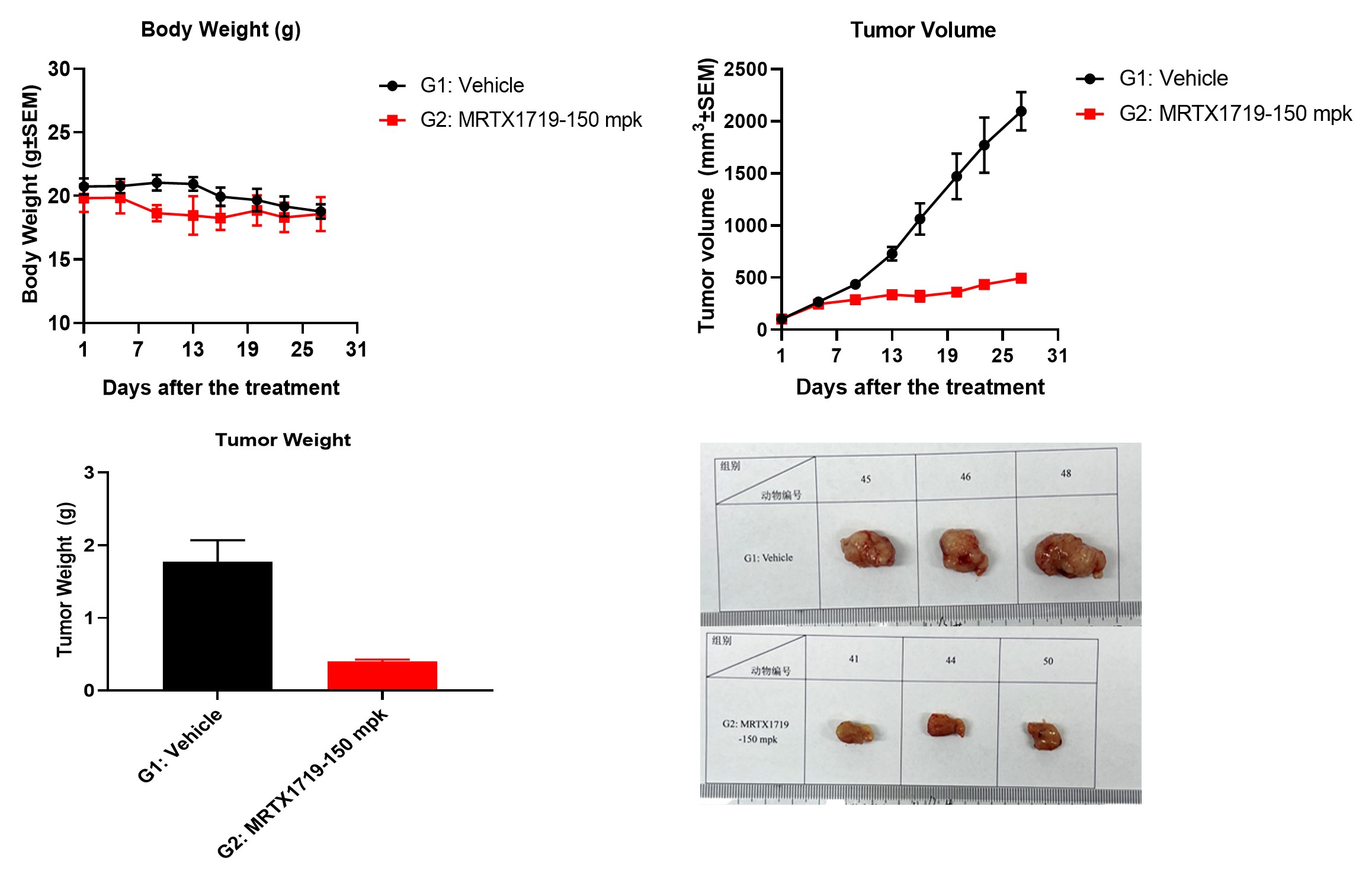
Figure 9. The in vivo efficacy of MRTX1719 in the HCT116-KO-MTAP CDX model.
This application note highlights our advanced capabilities in targeting the PRMT5/MEP50 complex, showcasing our expertise in both biochemical assays and biophysical analyses such as SPR, cell-based assays, and in vivo assays. By leveraging the MTase-Glo assay, we precisely quantify inhibitor efficacy across various conditions, including the presence of competitive cofactors like SAM and MTA. The cell-based assays and in vivo assays confirmed the inhibition of PRMT5 by the inhibitor MRTX1719. Our studies demonstrate not only the potency and selectivity of PRMT5 inhibitors like MRTX1719 and LLY-283 but also our ability to elucidate complex molecular interactions that are critical for optimizing therapeutic strategies. These insights underscore our strength in developing targeted therapies for PRMT5-related cancers, particularly those involving MTAP deletions, where precision and specificity are paramount.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.