The innate immune system serves as the body's first line of defense against pathogens, maintaining homeostasis through immune cells like monocytes, macrophages, dendritic cells, and neutrophils. The detection of pathogens is mediated through pattern recognition receptors (PRRs), which recognize pathogen-associated molecular patterns (PAMPs). Among the PRRs, Toll-like receptors (TLRs) play a central role in the immune response, initiating signaling cascades that promote inflammation and immune responses.
Interleukin-1 receptor-associated kinase 4 (IRAK4) is a critical kinase in the TLR and interleukin-1 receptor (IL-1R) signaling pathways. IRAK4 is essential for the assembly and signaling of the myddosome complex, which triggers downstream activation of nuclear factor-kappa B (NF-kB) and interferon regulatory factors (IRF 5/7). These factors lead to the release of pro-inflammatory cytokines and chemokines, including TNF-α, IL-6, IL-1β, and IL-23, which are known contributors to the pathogenesis of various autoimmune and inflammatory diseases, such as pyogenic tonsillitis, psoriasis, and atopic dermatitis.
Given its critical role in inflammatory signaling, IRAK4 has emerged as a promising target for therapeutic intervention aimed at attenuating TLR/IL-1R-mediated inflammation. The development of small molecule inhibitors and IRAK4-targeted protein degraders (PROTACs) is an area of significant interest in drug discovery.
HTRF Assays for Binary and Ternary Complex Formation: We utilize Homogeneous Time-Resolved Fluorescence (HTRF) to detect the formation of both binary (IRAK4-compound) and ternary (IRAK4-compound-E3 ligase) complexes. This enables the assessment of the binding affinity and specificity of both inhibitors and PROTAC candidates.
Cellular NanoBRET Assays: To confirm the formation of the ternary complex within cells, we deploy NanoBRET technology, allowing real-time monitoring of compound interactions and efficacy within a cellular context.
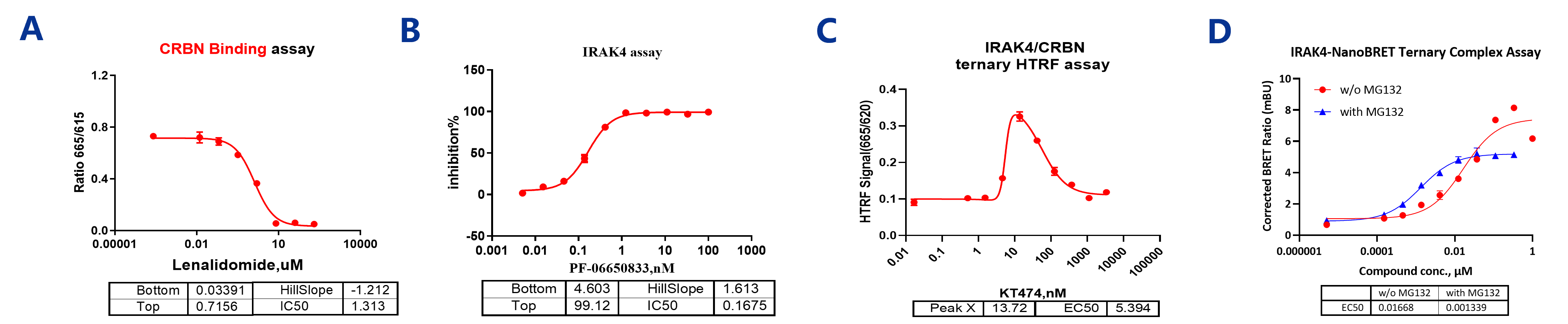
Biochemical and cellular assays for IRAK4 PROTAC activity. (A) CRBN binding measured by HTRF. (B) IRAK4 binding detected through HTRF. (C) Ternary complex formation between IRAK4, the compound, and CRBN using HTRF. (D) Cellular assessment of ternary complex formation using NanoBRET, showing dose-response curves with and without competitor.
IRAK4-HiBiT Assay: It utilizes a HiBiT-tagged IRAK4, a small and bright luminescent tag that can be detected upon binding to its complementary large BiT component, which is added during the assay. When IRAK4 is degraded in the presence of a test compound, the luminescence signal decreases, allowing for the direct measurement of the compound's degradation capacity and potency in cells. This rapid and sensitive assay provides insights into the degrader's efficacy.
Flow Cytometry: Human peripheral blood mononuclear cells (PBMCs), whole blood, or THP-1 cells are pre-treated with the compound for 24 hours, followed by intracellular labeling of IRAK4 for flow cytometry-based detection.
Western Blotting and ELISA: Degradation is further validated by protein quantification through Western blot analysis and ELISA, confirming the reduction in IRAK4 levels upon compound treatment.
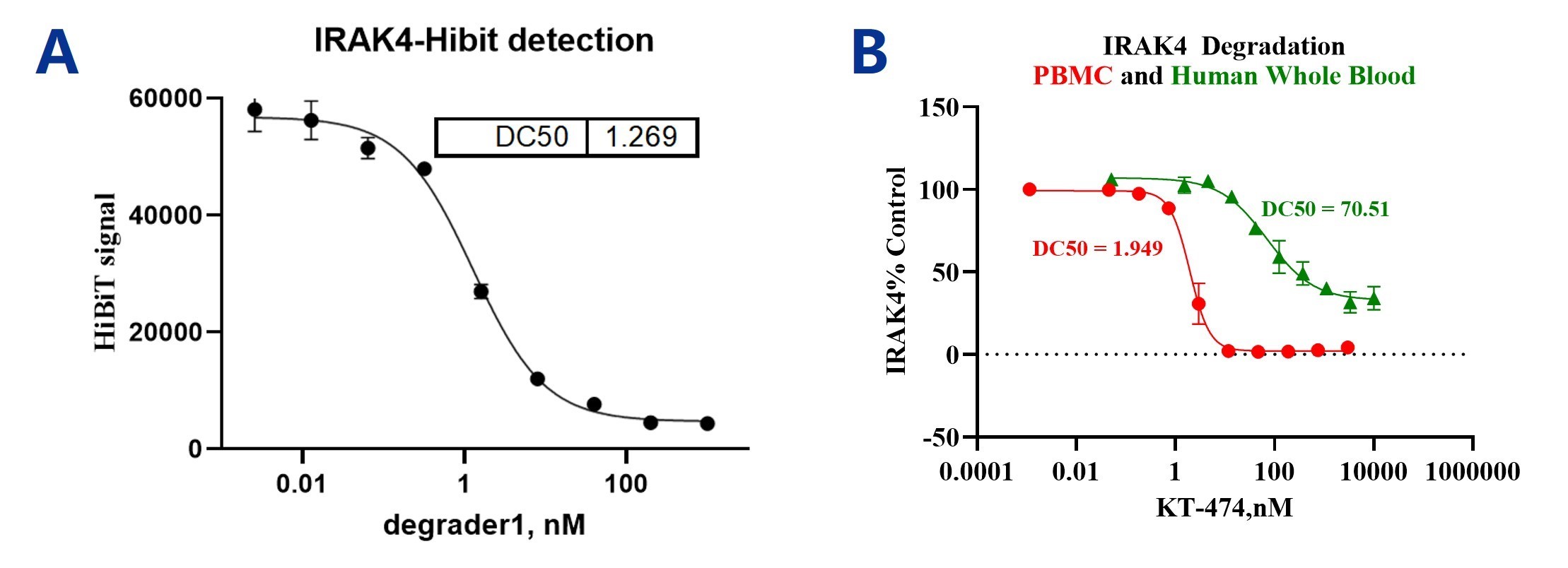
Assessment of IRAK4 degradation using various assays. (A) IRAK4-HiBiT cells were treated with the compound for 24 hours, and degradation was measured by detecting the HiBiT signal. (B) Human PBMCs and whole blood were pretreated with the compound for 24 hours, and intracellular IRAK4 was detected by flow cytometry.
Cytokine Secretion Analysis in Immune Cells: To assess the functional impact of IRAK4 inhibitors or PROTACs, we evaluate cytokine secretion in treated THP-1 cells, PBMCs, and whole blood. Compounds are pre-incubated (1 hour for inhibitors, 24 hours for PROTACs), followed by stimulation with TLR agonists like R848 or LPS. The resulting cytokine release is measured using ELISA, allowing for a detailed understanding of compound activity within the innate immune signaling context.
Whole Blood vs. Cellular Assays: Given that compound activity can differ significantly between isolated cells and whole blood, our assays include a comparative analysis to capture this variability and provide a more accurate prediction of in vivo behavior.
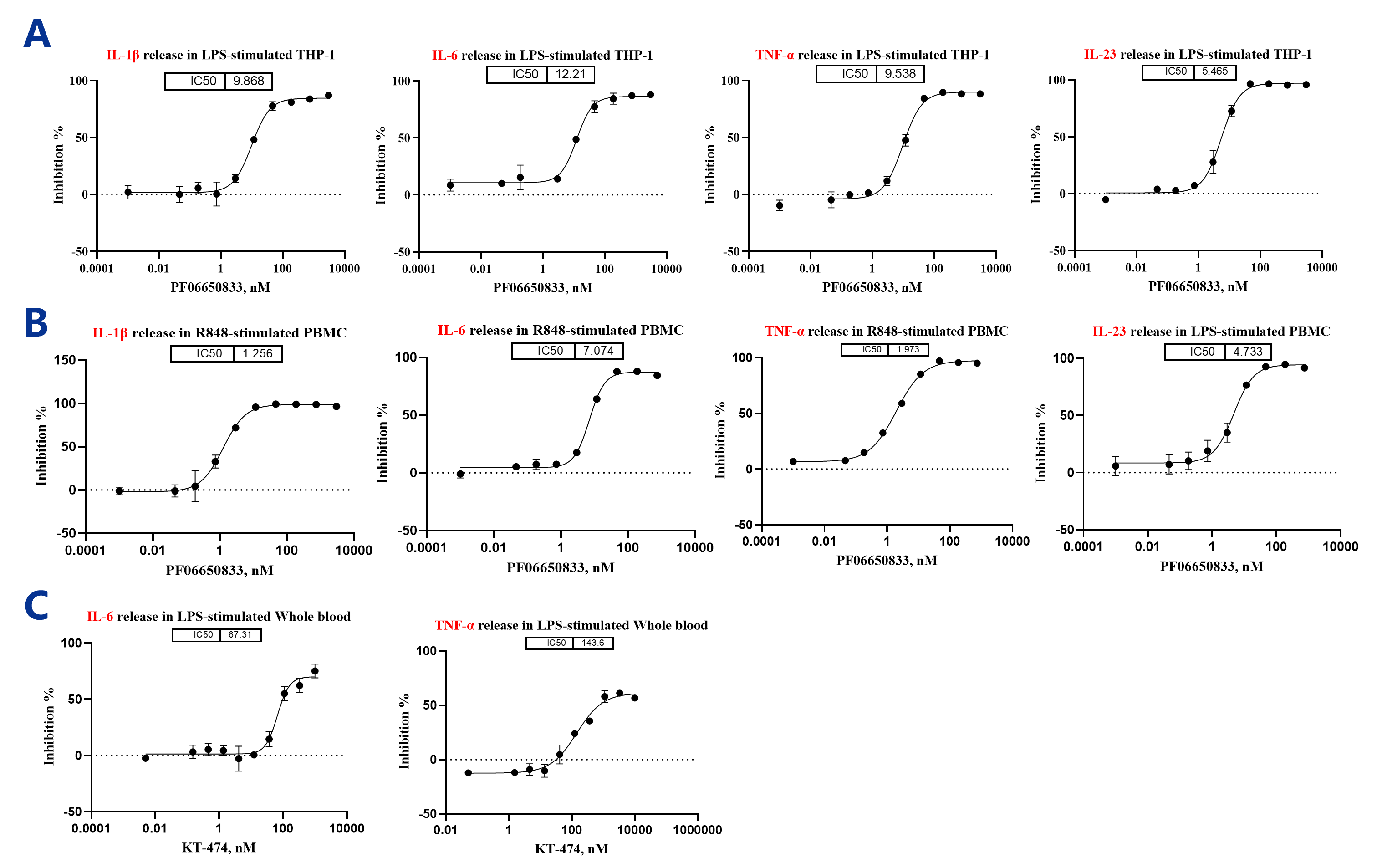
Functional assessment of IRAK4 inhibitor and PROTAC activity on cytokine secretion in different immune cell models. (A) THP-1 cells were treated with the compound, stimulated with R848/LPS, and cytokine levels (IL-1β, IL-6, TNF-α, IL-23) were measured. (B) Human PBMCs were pretreated with the compound, stimulated, and cytokine secretion was analyzed. (C) Human whole blood was treated similarly with PROTAC to evaluate cytokine modulation. Data demonstrates the effect of IRAK4 modulation on inflammatory cytokine release across cell models.
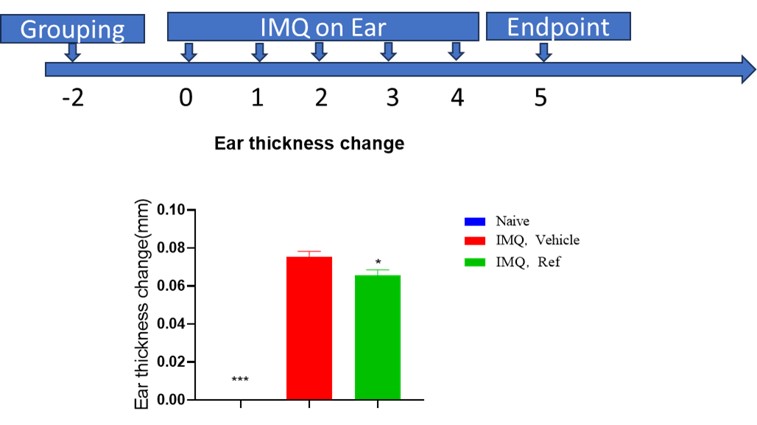
Psoriasis Model Using IMQ-Induced Th17 Activation: IRAK4's role in Th17 differentiation and function is crucial in the pathogenesis of psoriasis. We utilize an imiquimod (IMQ)-induced psoriasis-like model in mice, where IMQ application to mouse ears for 5-7 consecutive days induces skin thickening and scaling, mimicking psoriasis symptoms. IRAK4 PROTAC has demonstrated efficacy in reducing ear thickness, providing a robust preclinical assessment of therapeutic potential.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.