Apelin receptor (APJ) is a G protein-coupled receptor that is involved in the regulation of cardiovascular function, energy metabolism, and fluid homeostasis. Dysregulation of APJ has been associated with metabolic diseases such as obesity, diabetes, and heart failure, making it an important target for drug discovery. In the quest to develop therapeutics that target APJ activity, gaining a thorough understanding of its mechanisms and interactions is crucial for effectively tackling these widespread health concerns. The development of APJ-targeted therapies holds promise for improving treatment options for conditions linked to metabolic and cardiovascular dysfunction.
1. Spectral Shift Assay
The Dianthus Spectral Shift Assay is a label-free technique utilizing spectral scattering to detect small shifts in the spectrum under 590 nm excitation. It accurately determines Kd values at dual wavelengths of 650 nm and 670 nm. This assay is ideal for identifying low-affinity agonist ligands and weak molecular interactions. In the reference test, the Dianthus platform quickly detected the binding of Azelaprag to the APJ receptor (Kd 51.4 nM) in under 3 hours using high-throughput screening in 384-well plates. With the ready-to-use assay utilizing Dianthus spectral shift technology, we enhance the detection of receptor aggregation and improve the efficiency of drug screening for APJ drug candidates.
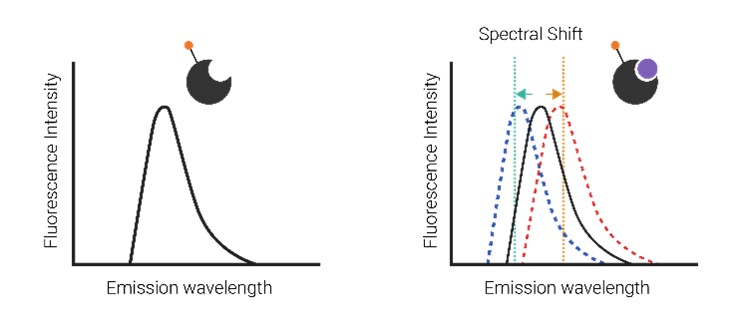
Figure 1. Comparative binding and activity profiles illustrating ligand-receptor interaction kinetics under varying conditions.
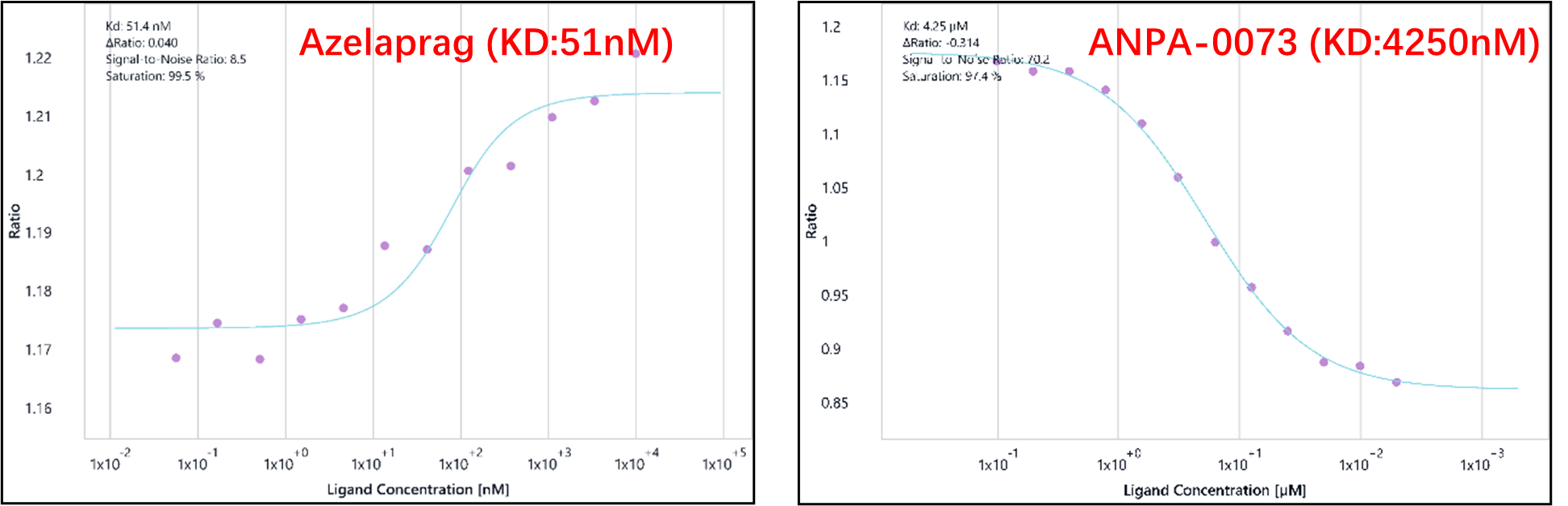
Figure 2. Dose-response curve for Azelaprag (balanced agonist) and ANPA-0073(biased agonist) with dissociation constant (KD) of 51.4 nM and 4250 nM, showing the binding affinity as a function of ligand concentration.
2. SPR Analysis
SPR (Surface Plasmon Resonance) analysis is an optical, label-free detection method widely used in molecular interaction studies. It captures molecular binding and dissociation by detecting changes in light reflection near a metal surface. When a ligand binds to an APJ receptor, the refractive index of the metal surface changes, altering the reflected light signal, which the instrument records in real time to generate binding kinetic curves. The reference test illustrates the 1:1 binding process curve of Azelaprag and APJ, showing response intensity and dissociation rates at varying concentrations. It also reveals the steady-state affinity curve of Azelaprag, indicating that the molecule has reached a steady-state after binding to APJ.
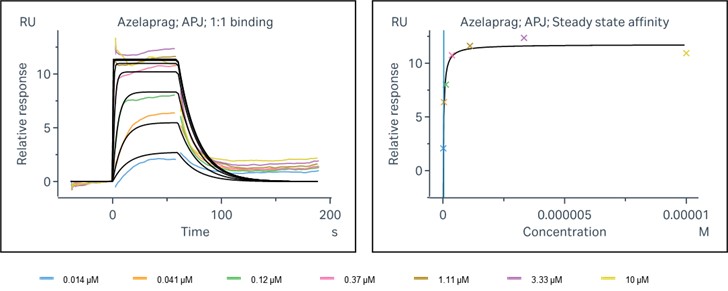
Figure 3. Binding and steady-state affinity analysis of Azelaprag with APJ receptor.
1. APJ-HTRF cAMP Assay
The APJ-HTRF cAMP Assay leverages Time-Resolved Fluorescence Resonence Energy Transfer (TR-FRET) technology to measure the levels of free cAMP and evaluate the impact of drugs on the APJ receptor. When Eu-labeled cAMP binds to ULight, it emits fluorescence at 665 nm, but in the presence of free cAMP, this FRET is blocked, resulting in fluorescence at 615 nm. CHO-Flp-In-APJ cells, which stably express the human APJ receptor, are used to evaluate APJ agonists Apelin-13 and Azelaprag, these two reference compounds induce cAMP production at low concentrations.
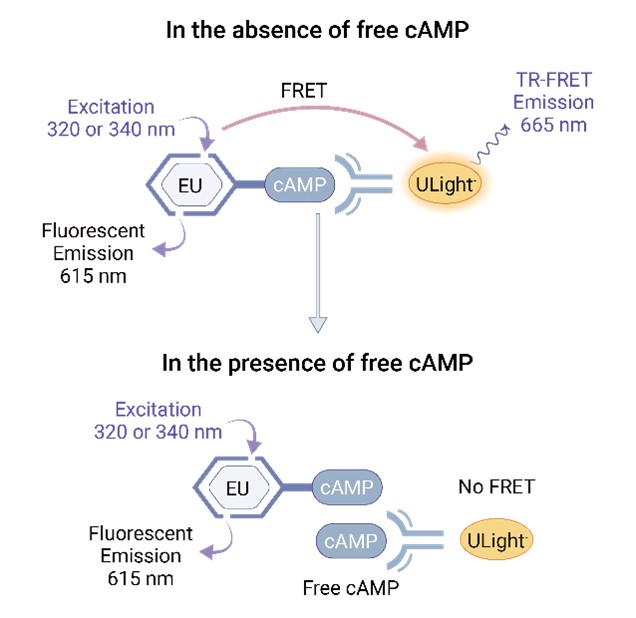
Figure 4. Schematic representation of TR-FRET assay for cAMP detection, illustrating excitation, emission, and energy transfer between Europium (EU) and ULight in the presence and absence of cAMP.

Figure 5. Dose-response curves for hAPJ receptor activation by Apelin-13 TFA, Azelaprag, and biased agonist ANPA0073.

Figure 6. Rat, mouse and dog APJ stable cells are ready to apply in compound species selectivity screening, apelin was used as reference in the validation.
2. APJ cAMP HTRF Assay: 1st Round of Mini Library Screening
Employing a 384-well plate setup, the platform is integrated with automated machinery such as the Echo 655 for dispensing compounds, MultiFlo and Multidrop instruments for seeding CHO-Flp-In-APJ cells and adding assay reagents, and the BMG FSX for HTRF ratio measurements. This setup enables the screening of approximately 1,000 compounds within a 2-day period.
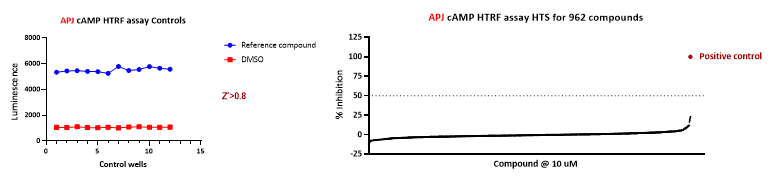
Figure 7. High-throughput screening of 962 compounds using the APJ cAMP HTRF platform. The process utilized automated systems for cell inoculation, compound addition, and data readouts, achieving a maximum inhibition rate of 23% in 2 days.
3. hAPJ β-Arrestin2 Recruitment Assay
The hAPJ/β-Arrestin2 NanoBiT assay utilizes stable HEK293T cell lines to visualize the recruitment of APJ and β-Arrestin2 through nano-luminescence technology, enabling sensitive detection of the Gi signaling pathway. Cells are cultured in 384-well plates and incubated for 2 minutes with Nano-Glo® substrates and compounds to assess receptor activity via luminescent signaling. The reference test demonstrated EC50 values for Apelin-13 TFA, Azelaprag and biased ANPA0073, with the latter showing different activity for the APJ receptor. This platform offers real-time dynamic detection, ease of use, broad applicability, and a short turnaround time, providing reliable data for drug screening and activity evaluation.
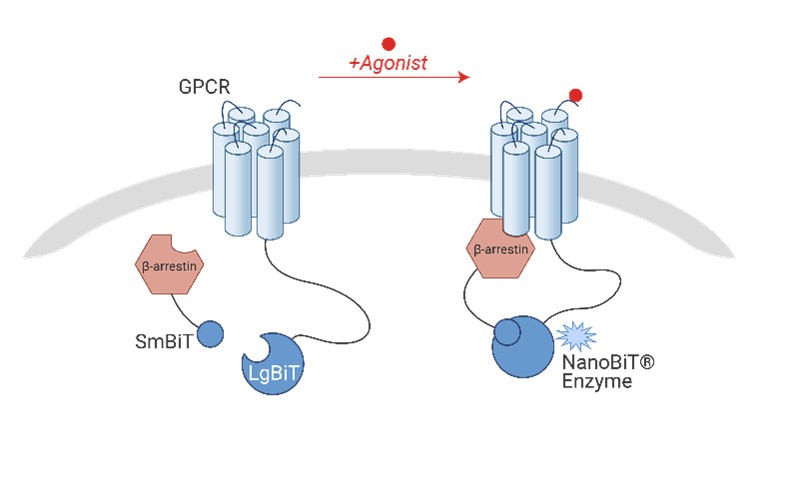
Figure 8. Schematic of GPCR β-arrestin recruitment assay using NanoBiT technology, illustrating the interaction between SmBiT and LgBiT upon agonist stimulation, leading to the formation of an active NanoBiT® enzyme.

Figure 9. Dose-response curves for APJ-Arrestin2 NanoBiT assay with Apelin-13 TFA, Azelaprag and biased agonist ANPA0073.
Choosing the appropriate assay is crucial for the efficient screening of APJ drug candidates. ICE Bioscience offers an all-encompassing platform that spans from biophysical to cell-based functional assays, delivering a holistic solution that boosts the efficiency and precision of APJ-targeted drug discovery through enhanced screening.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.