NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) is a member of the NOD-like receptor (NLR) family involved in immune responses. Its primary function is to act as a sensor, detecting danger signals to assemble and activate the NLRP3 inflammasome. The NLRP3 inflammasome complex consists of the NLRP3 sensor, the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD domain), and the effector protein caspase-1. As a key component of the innate immune system, the NLRP3 inflammasome plays a critical role in activating inflammatory responses and the onset of various inflammatory diseases. NLRP3 activation depends not only on its structural changes but also on interactions with other proteins. Among these, mitosis A (NIMA)-related kinase 7 (NEK7) is a key mediator of NLRP3 activation, directly binding to NLRP3 to promote its assembly and activation. Dysregulation of NLRP3 can lead to several inflammatory diseases, making it an important target for therapeutic intervention. As an essential component of the NLRP3 inflammasome, NEK7 is also an attractive target for inflammasome activation and related disease treatment. Currently, ICE Bioscience has developed a series of biochemical and cellular assays that allow for the study of NLRP3 activation, regulation, and its interactions with other proteins like NEK7. These tools provide important support for identifying potential drug candidates that can modulate NLRP3 activity and its role in inflammation.
1. NLRP3 ADP-Glo Assays
The NLRP3 ADP-Glo Assays detect the reaction of ATP hydrolysis to ADP by quantifying the luminescent signal, thereby assessing the ATPase activity of NLRP3. The ATPase activity of NLRP3 is mediated by its NACHT domain, which binds ATP and hydrolyzes it to ADP, driving conformational changes in the protein and activating its function. The intensity of the luminescent signal is proportional to the extent of ATP hydrolysis, reflecting the impact of inhibitors on NLRP3 activity. In the reference test, the NLRP3 ADP-Glo Assays were used to evaluate the inhibitory effect of MCC950 on NLRP3 ATPase activity. The results showed that MCC950's inhibition was dose-dependent, with an IC50 value of 22.08 nM, indicating that MCC950 effectively inhibits NLRP3 ATPase activity and supports its potential application as a drug candidate.
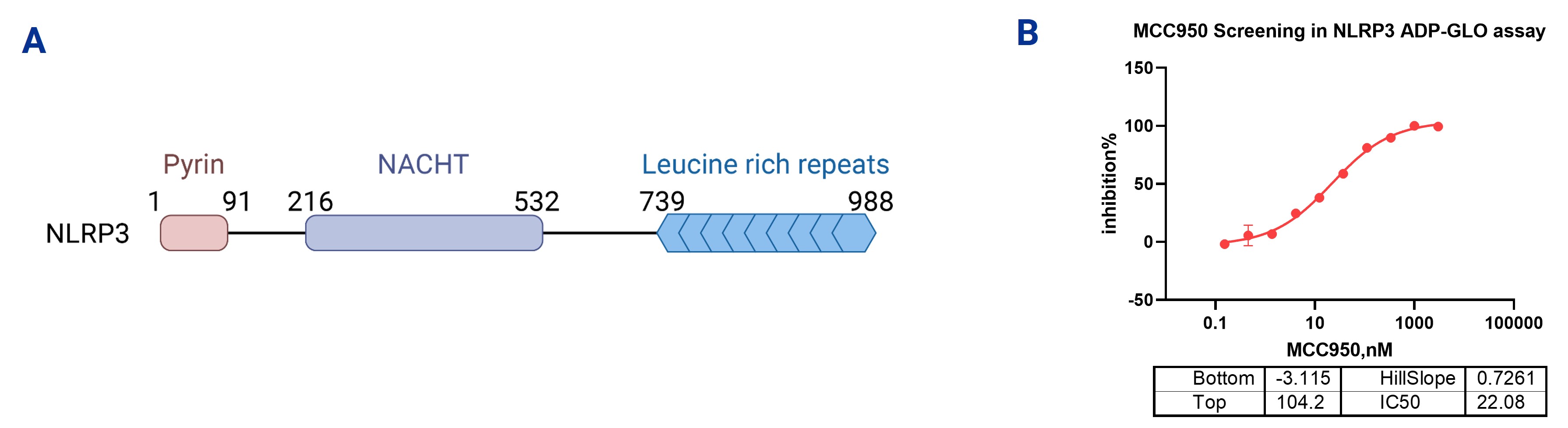
Figure 1. (A) The protein structure of NLRP3. (B) ADP-Glo assay was used to screen for the inhibition of NLRP3 ATPase enzyme activity by compounds.
2. NEK7 ADP-Glo Assays
The NEK7 ADP-Glo Assay is a sensitive enzyme activity detection method that evaluates the catalytic activity of NEK7 by monitoring the ADP levels generated during ATP hydrolysis. This technology is particularly useful for analyzing the role of NEK7 in NLRP3 inflammasome activation, as NEK7 plays a key regulatory role in this process. NEK7 consists of two domains: the N-lobe, which is responsible for binding to NLRP3 and promoting complex assembly, and the C-lobe, which has catalytic activity and is involved in ATP hydrolysis, directly influencing NLRP3 activation. In the reference test, the NEK7 ADP-Glo Assay was used to assess the inhibitory effect of Staurosporine on NEK7 enzyme activity. The experimental results indicated that Staurosporine effectively inhibited NEK7's ATP hydrolysis activity, suggesting that it may suppress NLRP3 activation by affecting the catalytic activity of the C-lobe of NEK7.
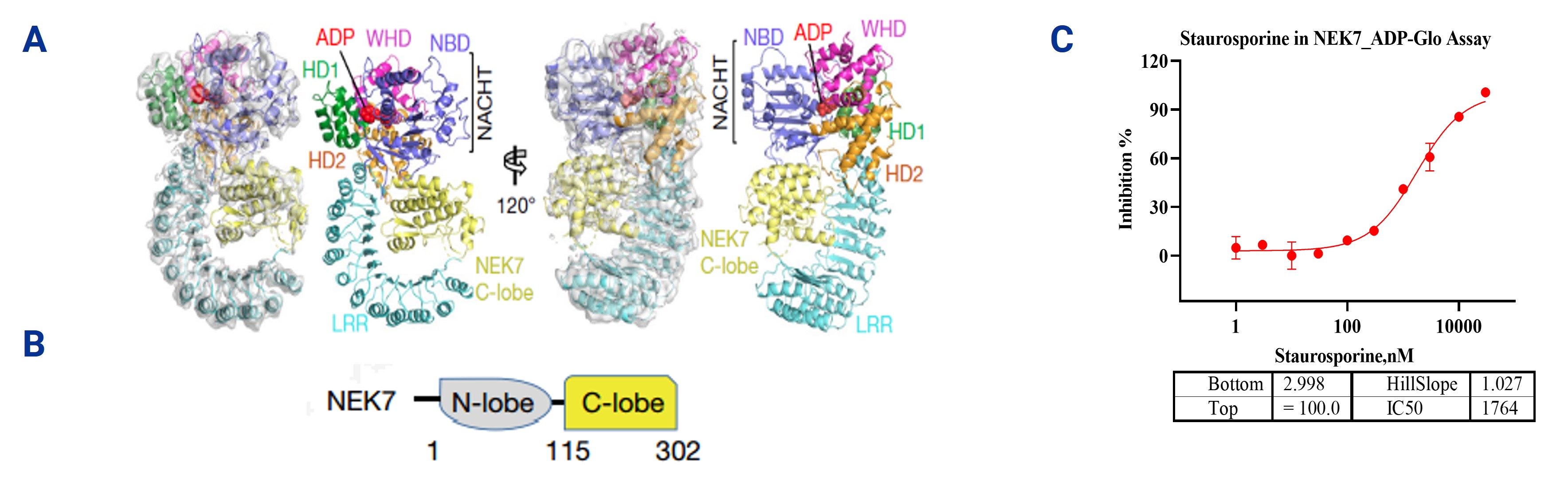
Figure 2. (A) The structure of the interaction between NEK7 and NLRP3 [1]. (B) The structure of NEK7. (C) ADP-Glo assay was used to screen for the inhibition of NEK7 enzyme activity by compounds.
3. NLRP3&NEK7 HTRF Assays
HTRF (Homogeneous Time-Resolved Fluorescence) assay can be used to analyze the interaction between NLRP3 and NEK7. This method utilizes the principle of fluorescence resonance energy transfer (FRET) to detect molecular interactions. When NLRP3 and NEK7 bind, the fluorescent probes that are labeled on each molecule transfer energy, generating a measurable fluorescence signal. Monitoring the changes in this signal can quantitatively assess the binding strength between the two. In the reference test, the experimental results showed that in the presence of 1mM ATP (physiological level), MCC950 exhibited significant inhibition of the binding between NLRP3 and NEK7, compared to the condition without ATP. This indicates that the HTRF assay can sensitively evaluate the regulatory effects of drugs on the activation process of the NLRP3 inflammasome, providing an important experimental basis for studying the mechanisms of inflammation and developing anti-inflammatory drugs.
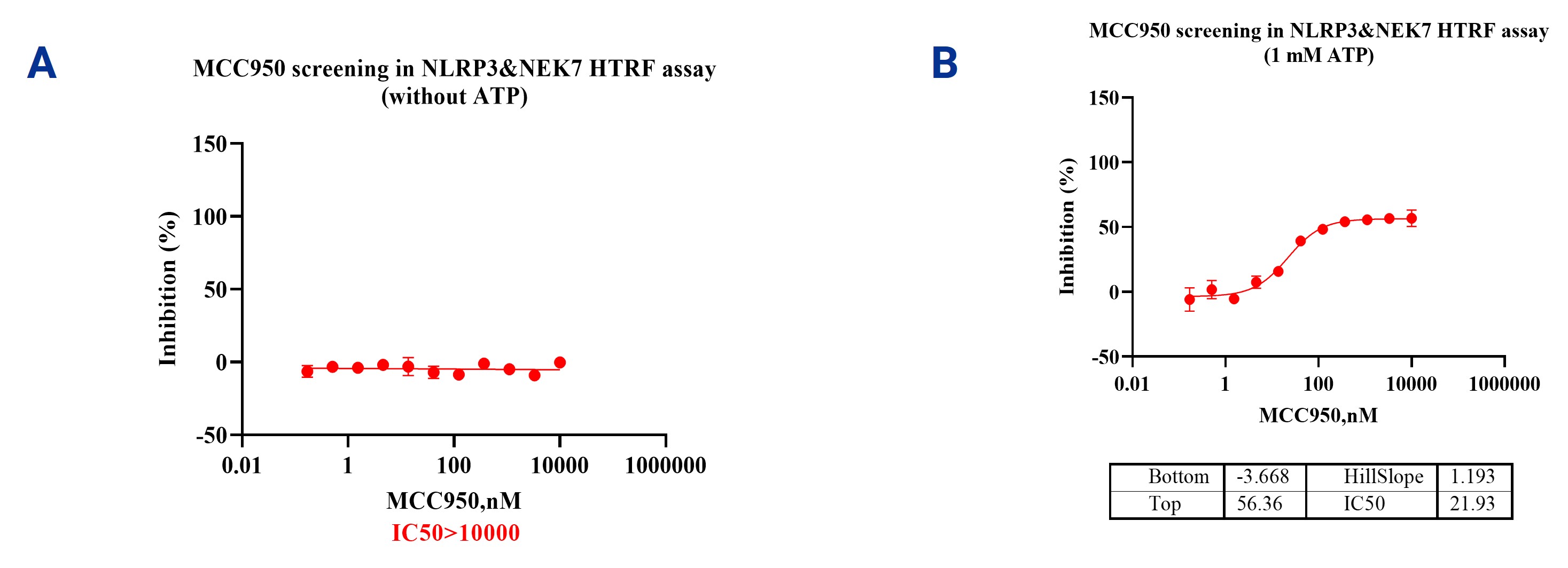
Figure 3. The effects of MCC950 on the interaction between NLRP3 and NEK7 without ATP (A) or with 1 mM ATP (B).
The NLRP3&NEK7 Cell-Based Assay can be used to study the activation and regulation of the NLRP3 inflammasome, as NLRP3 plays a key role in immune responses. In the assay, NLRP3 is first primed by lipopolysaccharide (LPS), and then further activated by stimulants such as ATP or Nigericin, leading to the activation of caspase-1, which promotes the maturation of inflammatory cytokines like IL-1β and IL-18, and triggers pyroptosis. NEK7 is a key mediator of NLRP3 activation, and inhibiting NEK7 (e.g., using MCC950) can effectively prevent inflammasome activation, reducing cytokine release and cell death. In the reference test, PMA-induced THP-1 cells, PBMCs, monocyte-derived macrophages, isolated primary microglial cells, human whole blood, and human macrophages were pretreated with LPS, then treated with MCC950 or NEK7 inhibitors, followed by the addition of Nigericin to measure cell viability and cytokine release. The results showed that with increasing concentrations of MCC950, cell viability increased, pyroptosis was inhibited, and the release of IL-1β and IL-18 was significantly reduced. Furthermore, MCC950 also effectively inhibited pyroptosis triggered by NLRP3 activation. These experiments indicate that using drugs like MCC950 or NEK7 inhibitors to block NLRP3 activation is important for evaluating the role of NLRP3 and NEK7 in inflammation and related diseases.
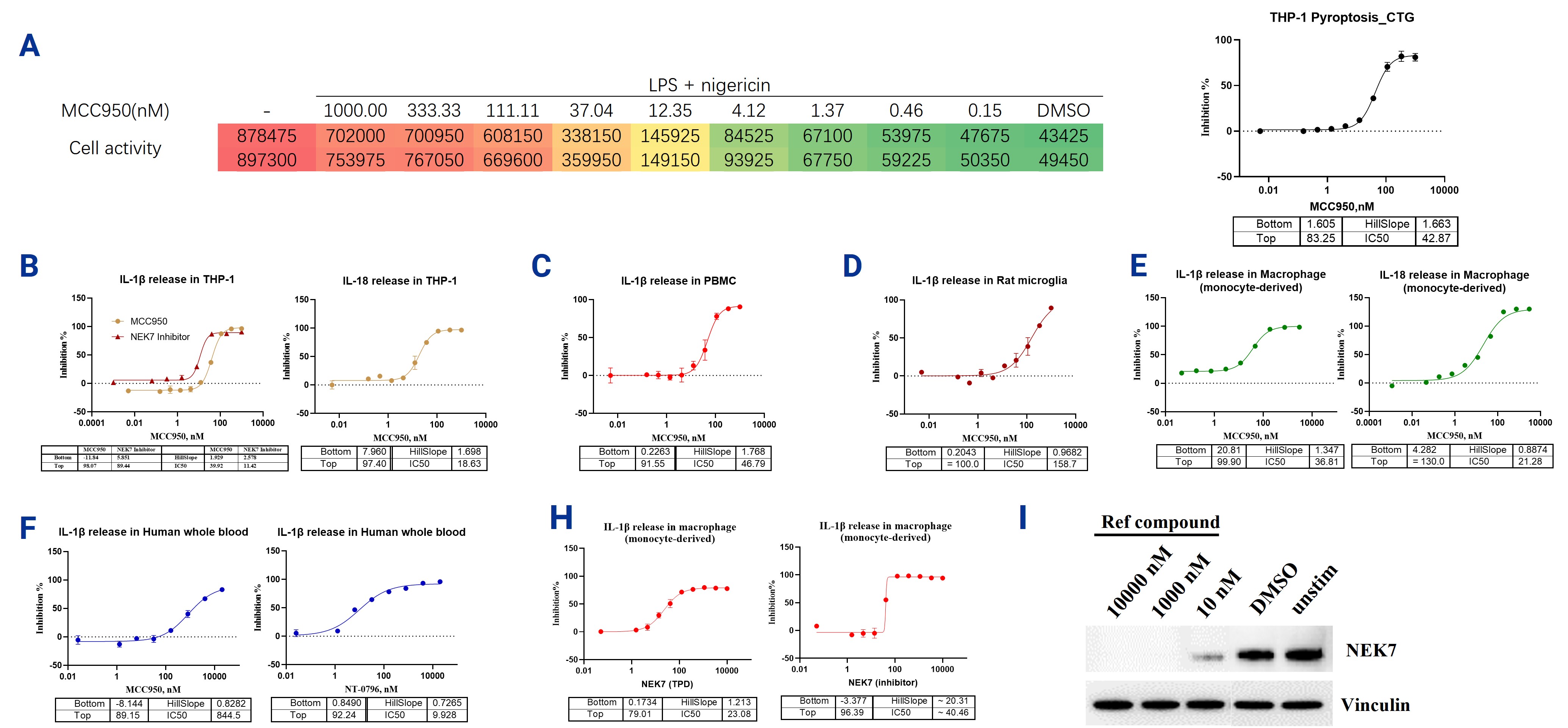
Figure 4. (A) THP-1 cells induced with PMA were utilized to assess NLRP3 or NEK7 inhibition and subsequent cell pyroptosis. (B) Release of IL-1β and IL-18 in THP-1 cells. (C) IL-1β release in peripheral blood mononuclear cells (PBMCs). (D) IL-1β release in rat microglia. (E) Release of IL-1β and IL-18 in monocyte-derived macrophages. (F) IL-1β release in human whole blood. (H) IL-1β release in human macrophage. (I) NEK7 degradation in human macrophage.
Selecting the right assay is essential for effective NLRP3 and NEK7 drug discovery. ICE Bioscience provides a comprehensive platform that includes both biochemical and cell-based assays, enabling in-depth study of NLRP3 activation and its interaction with NEK7. These assays, such as the NLRP3&NEK7 ADP-Glo Assays, NLRP3&NEK7 HTRF assays and cell-based assays, offer valuable insights into inflammasome regulation and drug impact, helping to identify potential therapeutic candidates. Through precise and reliable screening methods, ICE Bioscience enhances the efficiency and accuracy of NLRP3 and NEK7-targeted drug development, offering robust support for advancing anti-inflammatory research.
Reference
[1]. Sharif, H. et al. (2019) 'Structural mechanism for nek7-licensed activation of NLRP3 inflammasome’, Nature, 570(7761), pp. 338–343. doi:10.1038/s41586-019-1295-z.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.