STING (Interferon Gene Stimulating Factor) plays a key role in the intracellular DNA sensing pathway. cGAS senses pathogens to generate cGAMP, which binds to STING, translocates and oligomerizes, activates TBK1 and IRF-3, and ultimately induces type I interferons and inflammatory mediators. Sustained activation of cGAS-STING signaling is associated with autoimmunity, aging-associated inflammation, and neurodegenerative diseases, and thus precise regulation of its activity is essential for maintaining immune homeostasis. Based on this, pro-activators and inhibitors of cGAS/STING are potential drug targets that may provide new strategies in the anti-cancer, anti-pathogenic bacteria and anti-inflammatory fields. ICE Bioscience has established relevant in vitro screening platforms aimed at rapid screening of activators and inhibitors.
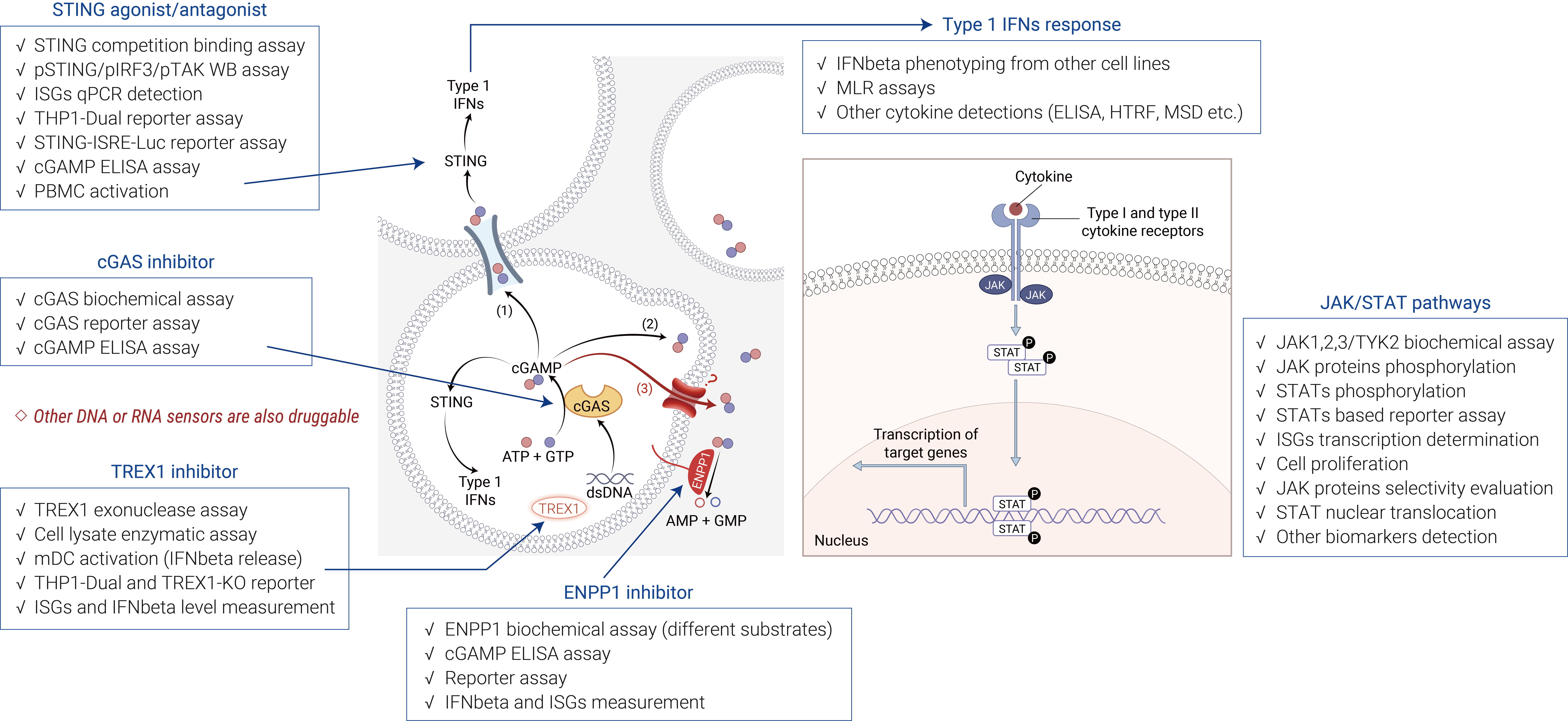
Figure 1. cGAS-STING pathway in and screening assay in ICE Bioscience
1. cGAS Antagonist/Agonist Reporter Assay
The cGAS Antagonist/Agonist Reporter Assay is a powerful tool for evaluating modulators of the cGAS-STING signaling pathway, which is critical for detecting cytosolic DNA and triggering immune responses. This assay employs reporter cells engineered to produce luminescence upon pathway activation, enabling the assessment of both agonists and antagonists. In the reference tests, the cGAS agonist G3-YSD significantly activated luminescence in both THP1-Dual and THP1-Dual-KI-hSTING-R232 cells. The effect was stronger in the hSTING-R232 variant, with a higher ECF value (2158 vs. 1068). Furthermore, the cGAS antagonist G150 effectively suppressed G3-YSD-induced activation in both cell lines, showing greater potency in hSTING-R232 cells (IC50 = 0.6507 µM vs. 0.9923 µM). These results illustrate the assay's sensitivity and reliability for studying the cGAS-STING pathway and underscore the importance of cell line optimization to enhance assay performance.
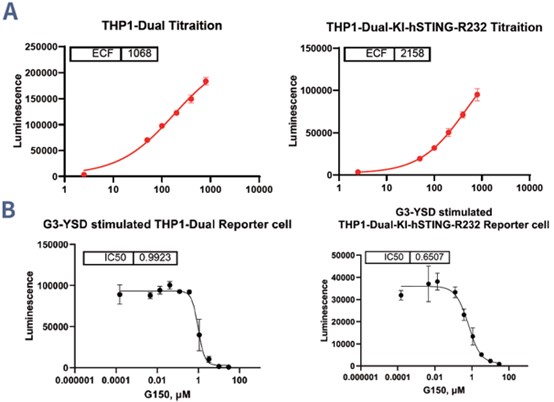
Figure 2. (A) The cGAS agonist G3-YSD elicits significant activation effects in THP1-Dual and THP1-Dual-KI-hSTING-R232 reporter cells. (B) The inhibitory effect of cGAS antagonist G150 on THP1-Dual and THP1-Dual-KI-hSTING-R232 reporter cells activated by G3-YSD was observed.
2. STING Atagonist/Agonist Reporter Assay
STING Agonist/Antagonist Reporter Assay is a key tool for investigating the activation and inhibition of the STING pathway, which plays a crucial role in immune responses to cytosolic DNA. In this assay, reporter cells produce luminescence in response to STING activation, allowing researchers to measure the pathway's activity. In the reference tests, the STING agonists 2',3'-cGAMP and diABZI effectively activated the pathway in THP1-Dual-KI-hSTING-R232 cells, with diABZI showing a stronger activation. Additionally, the STING antagonist H151 successfully inhibited STING activation induced by both 2',3'-cGAMP and diABZI, demonstrating its effectiveness in blocking the pathway. This assay provides a reliable platform to study the modulation of STING signaling, offering insights into immune regulation and potential therapeutic interventions.
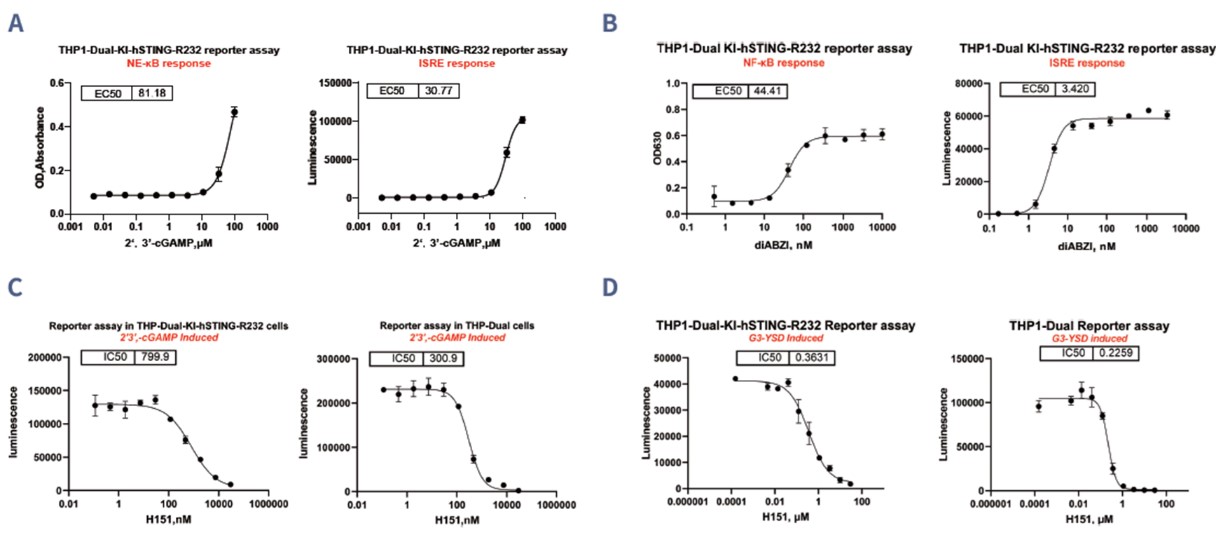
Figure 3. Activation of STING by (A) 2',3'-cGAMP and (B) diABZI in THP1-Dual-KI-hSTING-R232 reporter cells. Inhibition of STING stimulated by (C) 2',3'-cGAMP and (D) G3-YSD by H151 in THP1-Dual-KI-hSTING-R232 reporter cells.
3. cGAS Pathway: Cytokine Release Assay
The Cytokine Release Assay is essential for studying the regulation of cytokine production, particularly IFN-β, via the cGAS signaling pathway. This assay utilizes THP-1 cells or human PBMCs to evaluate the immunomodulatory effects of compounds. In the reference tests, the cGAS agonist G3-YSD effectively activated IFN-β release in a dose-dependent manner, demonstrating its strong pathway activation capability (Figure 4A). Conversely, the cGAS antagonist G150 showed significant inhibition of G3-YSD-induced IFN-β production, with a clear dose-dependent effect (Figure 4B). These findings demonstrate the assay’s ability to assess both activation and inhibition of the cGAS pathway, providing a reliable platform for identifying compounds that modulate immune responses and offering potential insights for therapeutic development.
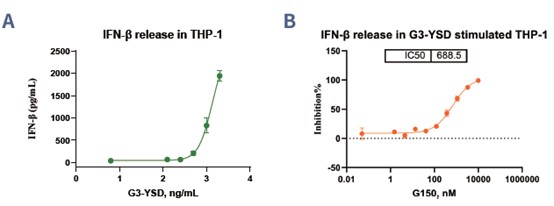
Figure 4. Data on the impact of different compounds, G3-YSD (A) and G150 (B) inactivating/inhibiting the release of IFN-β in THP-1 cells.
4. STING Pathway: Cytokine Release Assay
The Cytokine Release Assay is a key method for evaluating STING pathway activation and its impact on IFN-β production in immune cells like human PBMCs and THP-1 cells. In this assay, cells are stimulated with STING agonists, and cytokine release is measured to assess immune activation. In the reference tests, 2',3'-cGAMP induced a dose-dependent increase in IFN-β release in both human PBMCs and THP-1 cells (Figure 5A). Similarly, diABZI also activated the STING pathway, resulting in significant cytokine production in both cell types (Figure 5B). These results demonstrate the assay's effectiveness in screening STING agonists and studying immune modulation, making it a valuable tool for immune response research and therapeutic development.
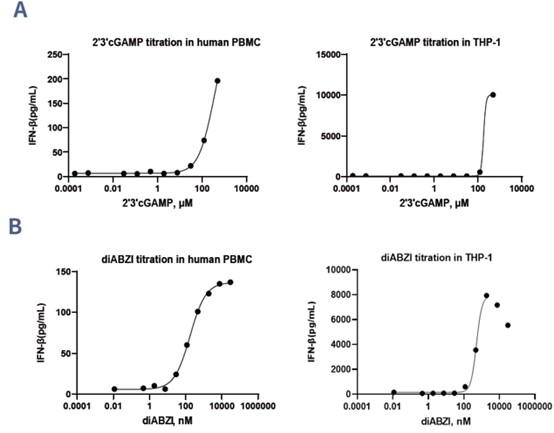
Figure 5. Data on activity of different agonist, 2',3'-cGAMP (A) and diABZI (B) in activating the release of IFN-β in human PBMC and THP-1 cells.
Western Blot Assay of STING
Western blot (WB) assays are crucial for studying the cGAS-STING pathway, allowing the detection of key biomarkers like STING oligomerization, phosphorylation of STING, TBK1, and IRF3. Upon cGAMP stimulation, STING undergoes phosphorylation and oligomerization, processes that can be analyzed using WB, native PAGE WB, and digital WB techniques like JESS. In the reference tests with H151, WB and native PAGE WB analysis of cGAMP-stimulated THP-1 cells revealed significant inhibition of pSTING and pIRF3, along with STING oligomerization, indicating the activity of the inhibitor. Additionally, JESS analysis of RAW264.7 cells confirmed a dose-dependent decrease in pSTING levels with H151. These findings demonstrate the effectiveness of WB and Jess automated western assays in studying the molecular mechanisms of the cGAS-STING pathway, providing valuable insights into immune response regulation.
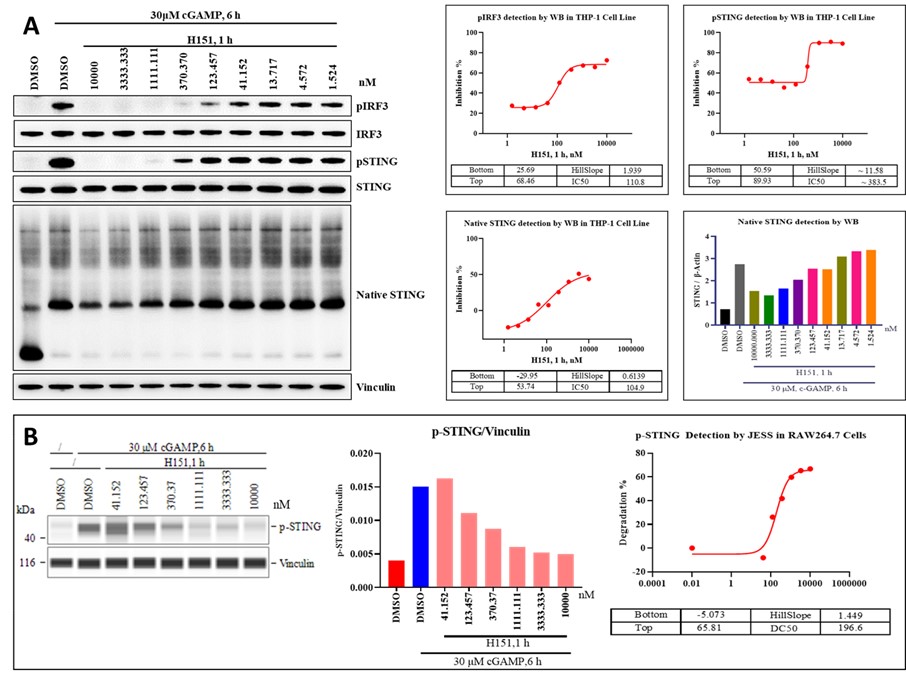
Figure 6. (A) WB assay of pSTING, pIRF3, Native page WB of STING oligomeriztion in cGAMP stimulated THP-1 cells. (B) JESS analysis of pSTING in cGAMP stimulated RAW264.7 cells.
The cGAS-STING pathway is critical for immune responses to intracellular DNA, and its dysregulation is associated with autoimmune diseases, inflammation, and cancer. Cellular screening platforms help identify modulators of this pathway for therapeutic development. The cGAS and STING Reporter Assays evaluate pathway activation and inhibition through luminescence, while cytokine release assays measure IFN-β production. Western blot and digital WB assays, like JESS, provide detailed insights into protein phosphorylation and STING oligomerization. These tools are invaluable for studying pathway mechanisms. ICE Bioscience has established advanced in vitro platforms to rapidly screen cGAS/STING activators and inhibitors, supporting research and therapeutic innovation.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.