Voltage-gated sodium channel Nav1.8 plays a key role in peripheral pain signal transmission and is a proven target in the development of non-opioid analgesics. Tetrodotoxin-resistant (TTX-R) sodium currents in dorsal root ganglion (DRG) neurons are primarily mediated by Nav1.8 and represent a critical readout for assessing analgesic compounds.
While high-throughput screening using human Nav1.8-expressing cell lines provides valuable potency data at the target level, these systems lack the complex physiological environment of native sensory neurons. Cynomolgus monkey DRG neurons, as a non-human primate model with high genetic similarity to humans, offer a critical bridge for validating analgesic candidates in a more biologically relevant context, particularly when human primary DRG neurons are not available.
We evaluated the Nav1.8 inhibitor VX-548 across multiple species using whole-cell patch-clamp recordings to assess inhibition of TTX-R currents under both resting (TP1) and half-inactivated (TP2) channel states.
Tested models included:
✅ Human Nav1.8-CHO stable cell line
✅ Rat DRG neurons
✅ Mouse DRG neurons
✅ Cynomolgus monkey DRG neurons
Cross-Species Comparison of VX-548 Potency
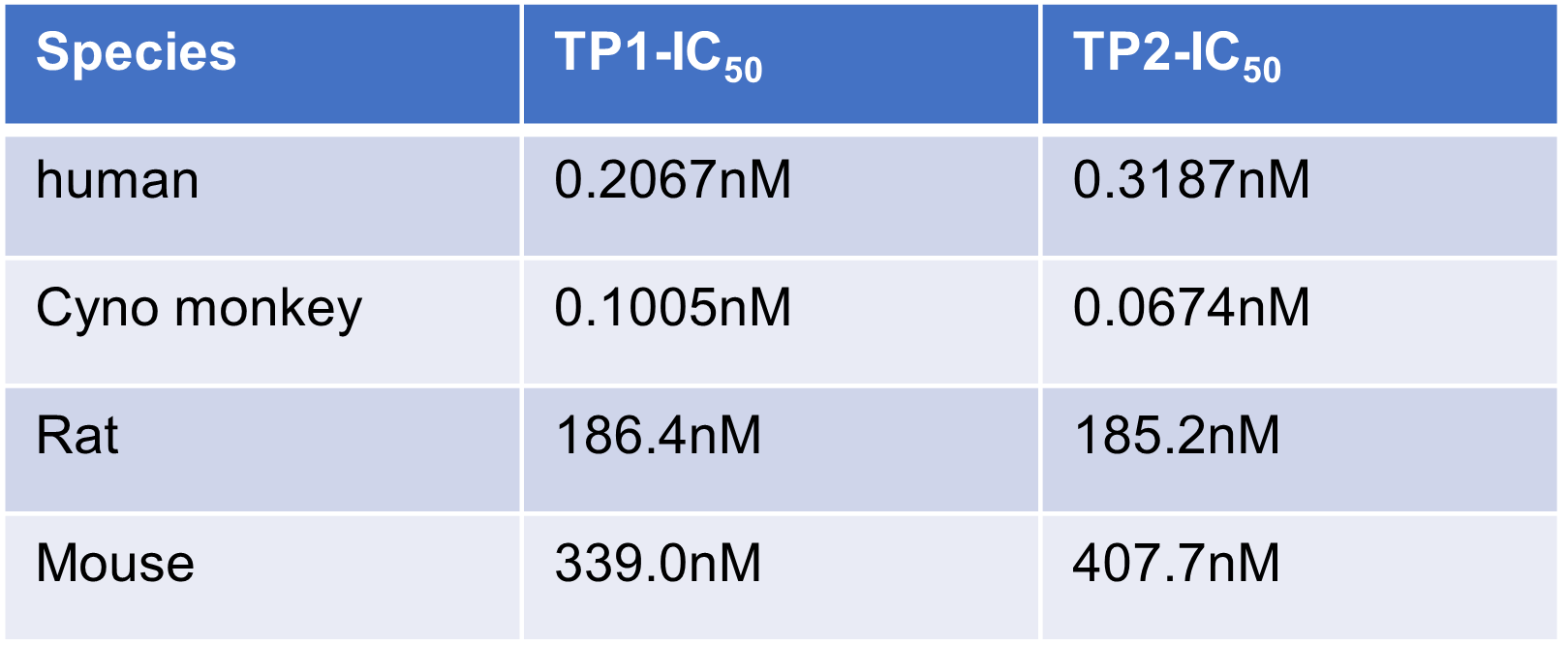
TP1: Resting state; TP2: Half-inactivated state.
These results demonstrate that Cynomolgus monkey DRG neurons demonstrated sub-nanomolar Nav1.8 inhibition potency similar to that observed in human Nav1.8-expressing cell lines, supporting their use as a translational model to confirm compound activity in native neuronal systems.
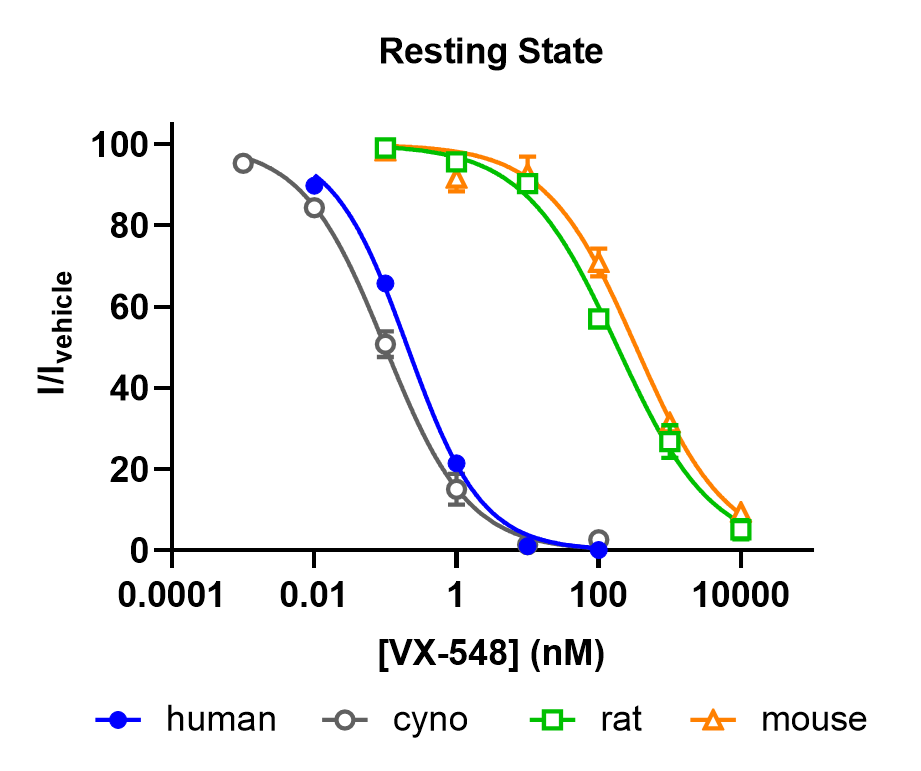
Figure 1. Concentration-dependent effect of VX-548 on human Nav1.8-CHO stable cell line, rat and mouse DRG neurons TTX-R currents in resting state.
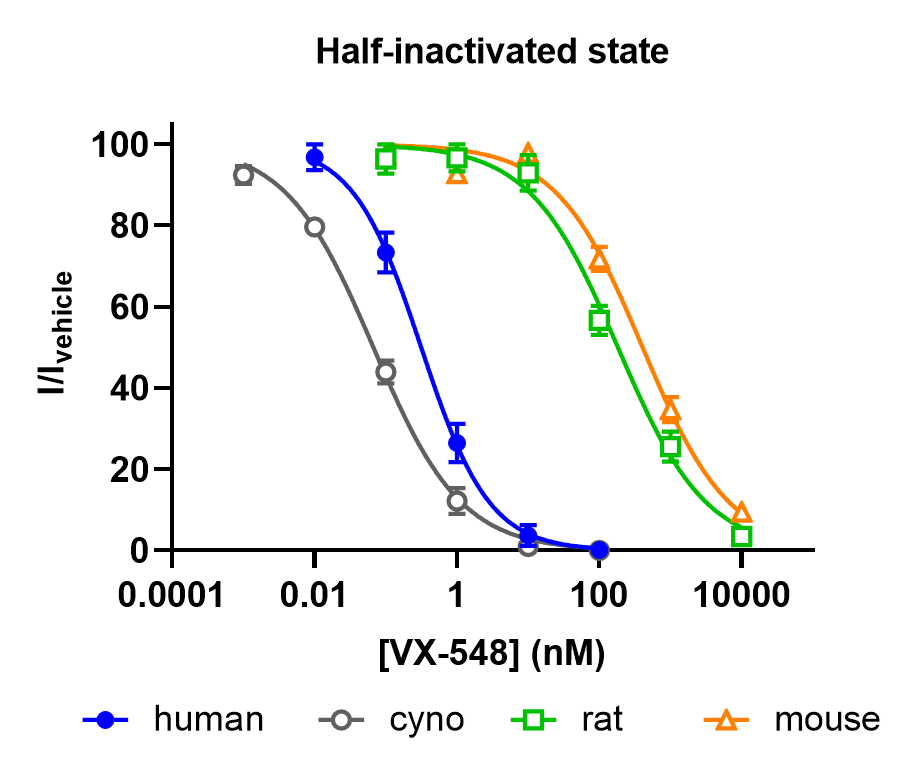
Figure 2. Concentration-dependent effect of VX-548 on human Nav1.8-CHO stable cell line, rat and mouse DRG neurons TTX-R currents in half-inactivated state.
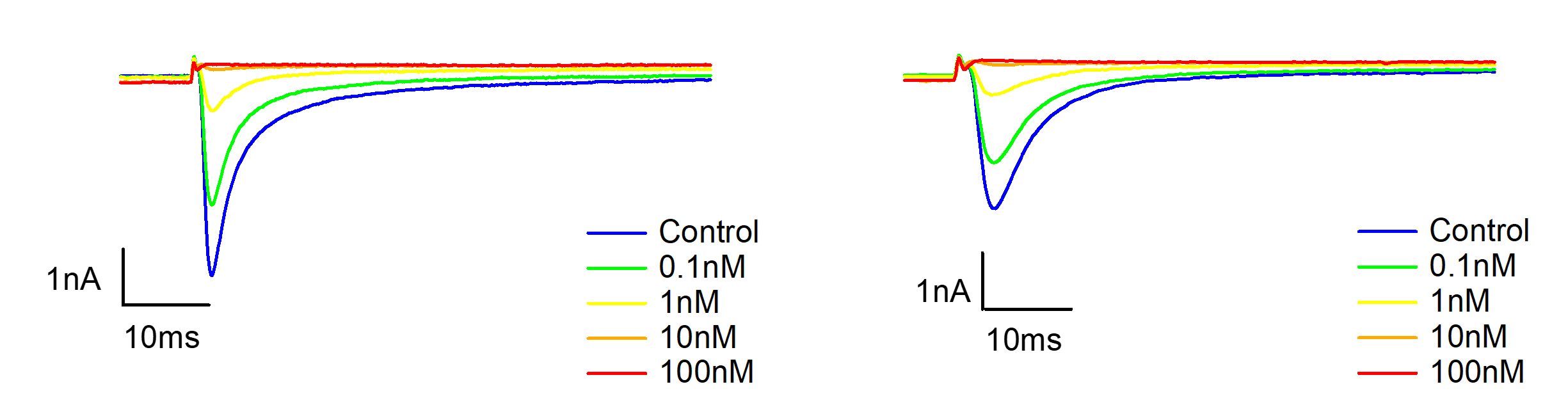
Figure 3. Representative currents of human Nav1.8-CHO stable line, before and after VX-548 application at different concentrations in resting and half-inactivated state.
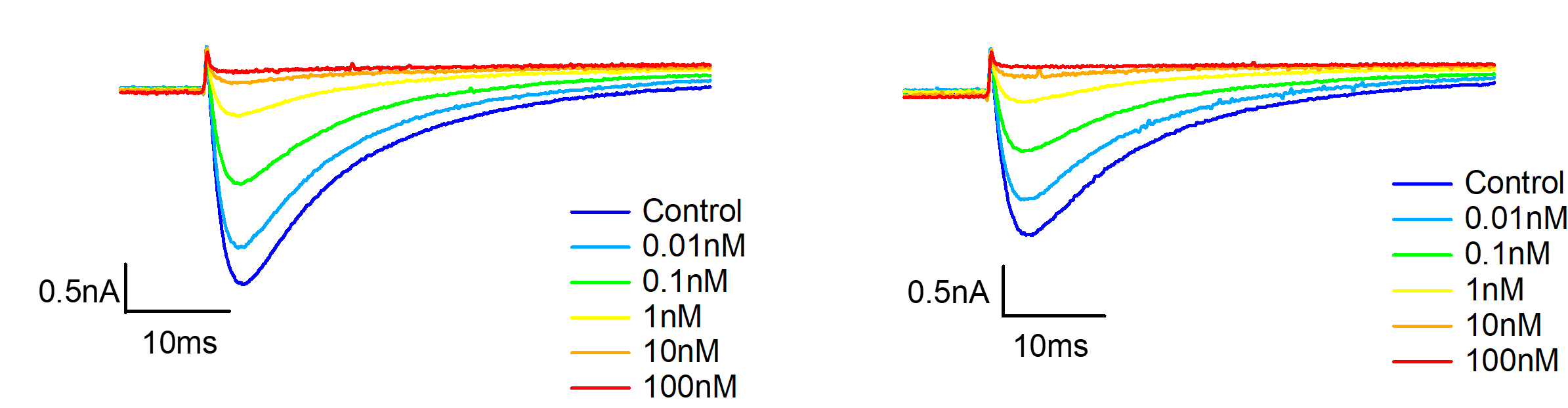
Figure 4. Representative currents of cyno DRG neurons TTX-R channel, before and after VX-548 application at different concentrations in resting and half-inactivated state.
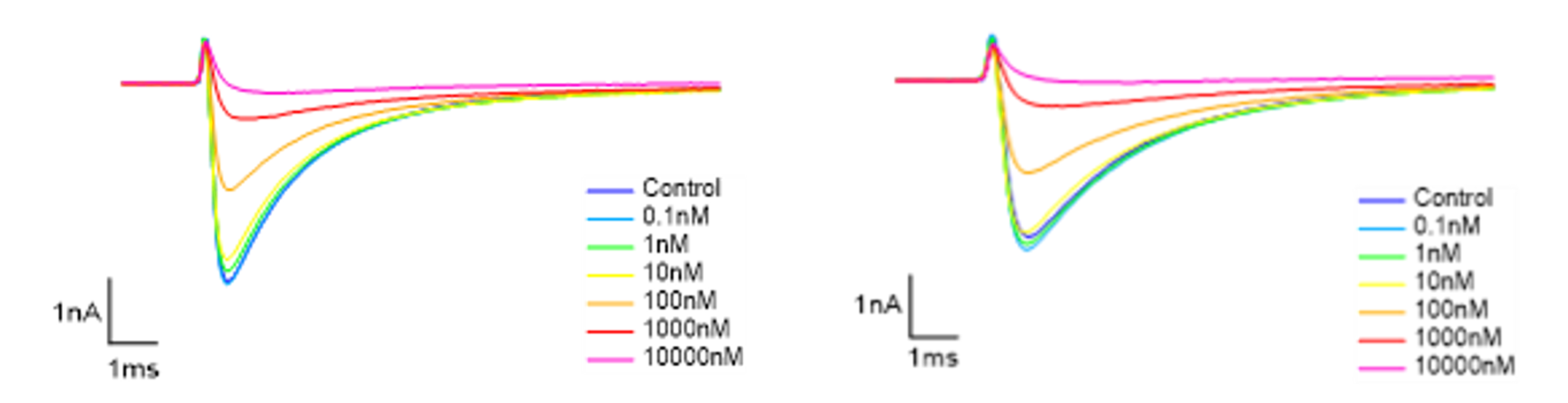
Figure 5. Representative currents of rat DRG neurons TTX-R channel, before and after VX-548 application at different concentrations in resting and half-inactivated state.
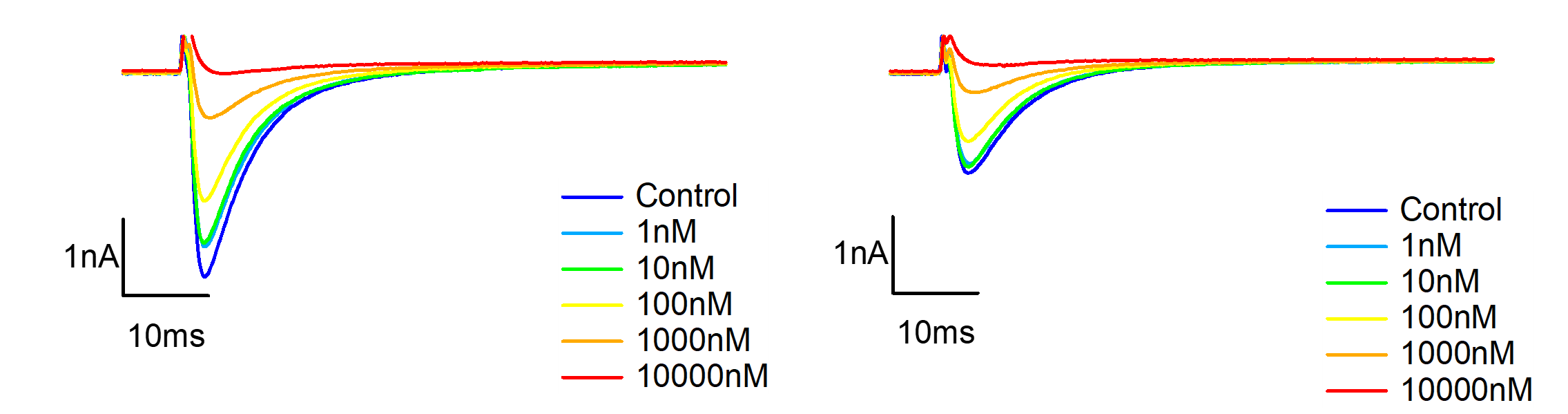
Figure 6. Representative currents of mouse DRG neurons TTX-R channel, before and after VX-548 application at different concentrations in resting and half-inactivated state.
To further demonstrate the value of cynomolgus monkey DRG assays, Compound X was tested for its ability to inhibit TTX-R sodium currents.
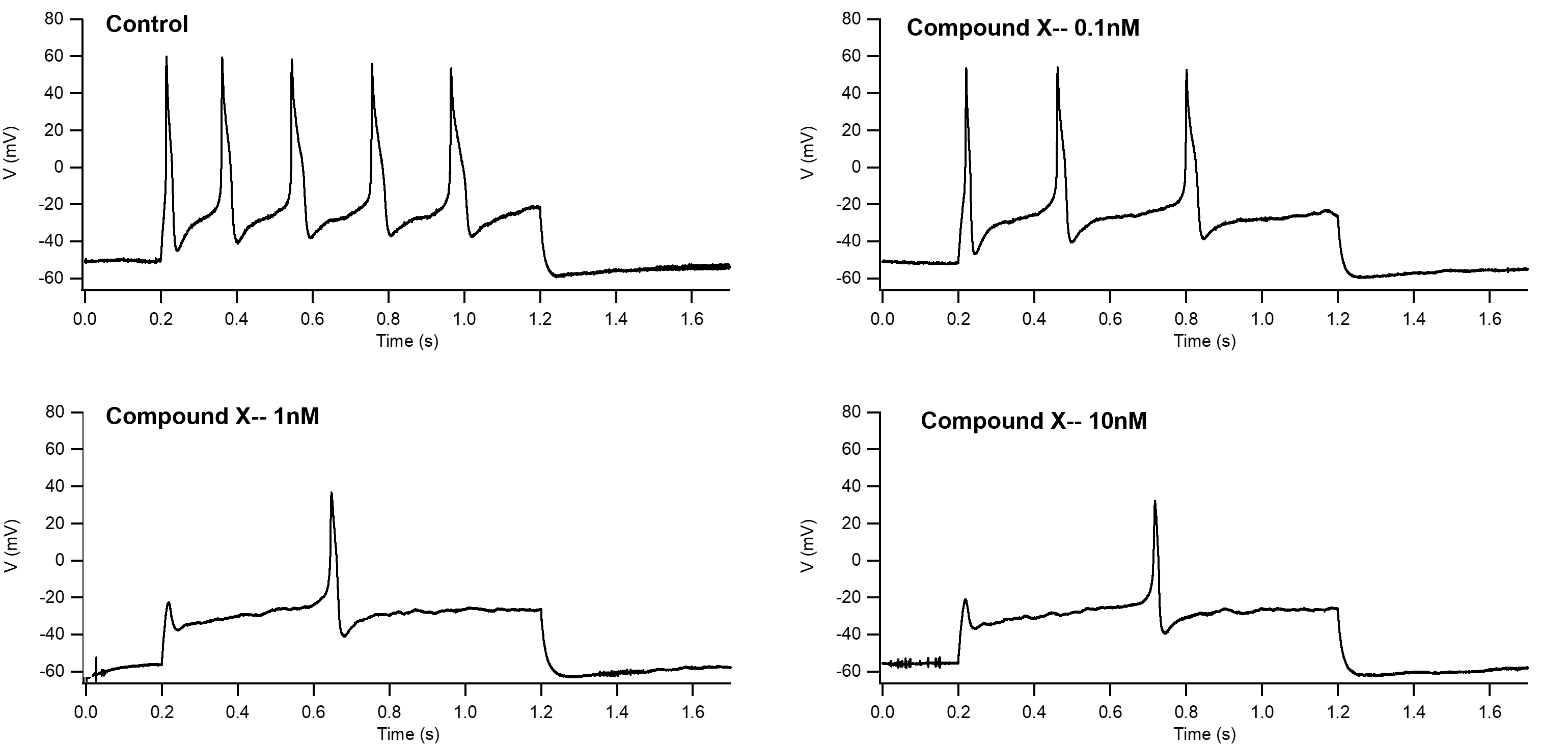
Figure 7: Concentration-dependent inhibition of TTX-R currents by Compound X in cynomolgus monkey DRG neurons.
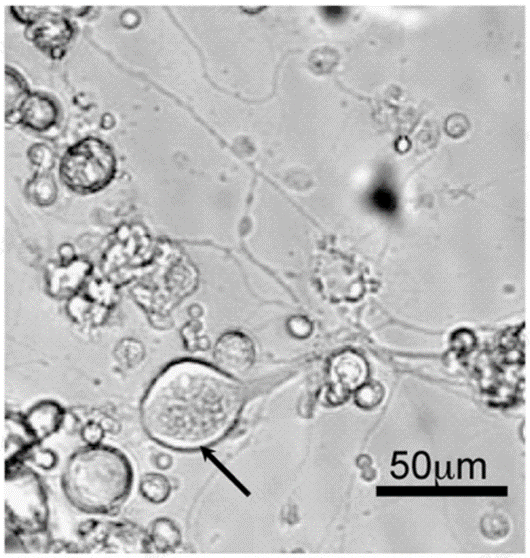
Figure 8. Representative image of cultured DRG neurons from cynomolgus monkey. The arrow indicates a typical DRG neuron.
To further demonstrate the value of cynomolgus monkey DRG assays, Compound X was tested for its ability to inhibit TTX-R sodium currents.
✅ Identifying potent Nav1.8 inhibitors
✅ Bridging preclinical and clinical pharmacology
✅ Supporting dose selection and risk assessment
Cynomolgus monkey DRG neurons provide a translational model for validating Nav1.8-targeted analgesics beyond recombinant systems. While human Nav1.8-expressing cell lines are essential for early-stage screening, cyno DRG assays allow confirmation of compound activity in native sensory neurons, offering greater physiological relevance.
By integrating cyno DRG electrophysiology into the screening workflow, researchers can enhance the predictability of preclinical data and better support the transition to clinical development in pain therapeutics.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.