Following our initial in vitro evaluation of a VAV1 molecular glue degrader (Part 1), this follow-up study showcases additional data to support its preclinical development. We highlight in vivo degradation in mouse models, mechanism of action studies, and profiling including in vitro ADME, hERG, and safety panel assays with a non-disclosed VAV1 degrader compound—demonstrating the breadth of our platform to accelerate degrader programs from early discovery to preclinical readiness.
To validate degrader activity in vivo, we assessed VAV1 protein levels in mouse whole blood and spleen following oral dosing. Degradation was confirmed by both flow cytometry and Jess immunoassay, demonstrating effective target engagement in peripheral and lymphoid tissues.
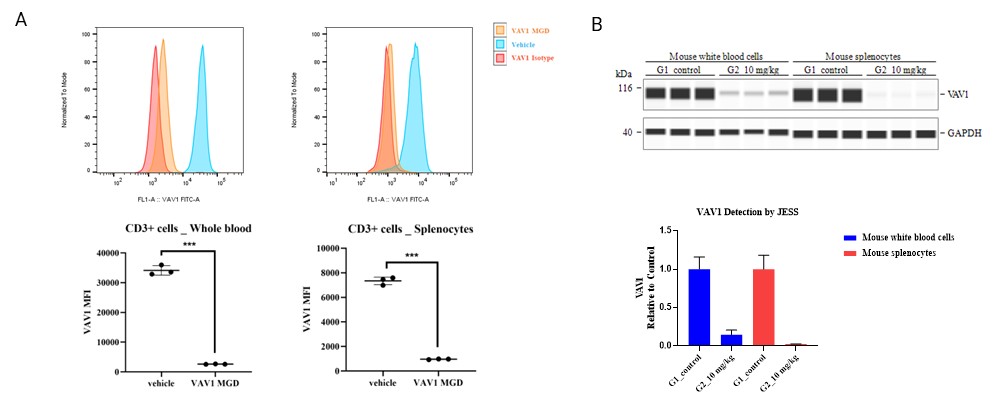
Figure 1. In vivo degradation of mouse VAV1. (A) Flow cytometry detection of VAV1 levels in CD3+ T cells from mouse whole blood and splenocytes 24 hours after oral dosing with VAV1 MGD. (B) Jess-based quantification of VAV1 in mouse samples.
We provide rapid, multi-angle analysis of degradation mechanisms using CHX chase, time-course assays, and pathway validation to confirm proteasome dependency.
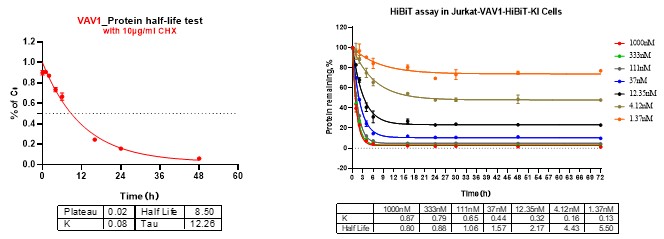
Figure 2: VAV1 undergoes dose- and time-dependent degradation. Left: CHX chase assay confirms active protein turnover. Right: Time- and dose-dependent degradation kinetics of VAV1 measured by HiBiT assay.
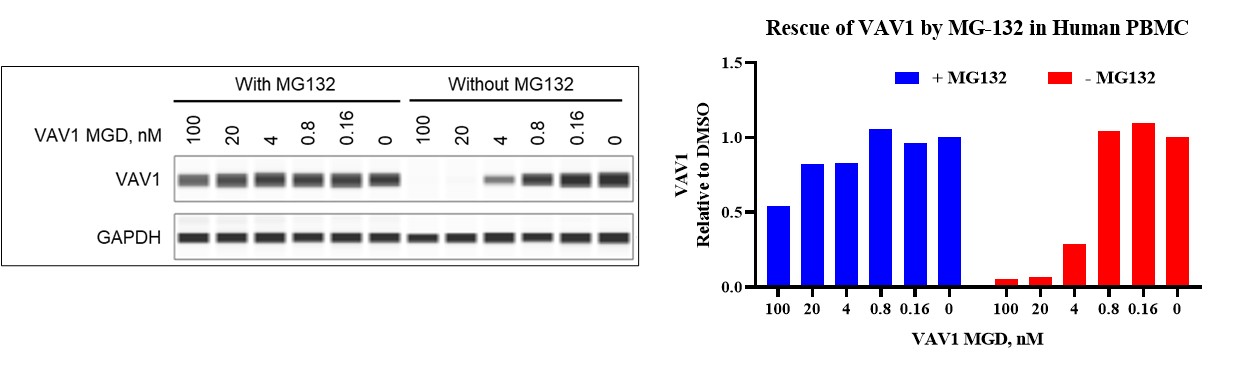
Figure 3: Treatment with the VAV1 MGD induces degradation of VAV1 protein, which is rescued by the proteasome inhibitor MG132. This indicates that VAV1 is degraded via a proteasome-dependent mechanism.
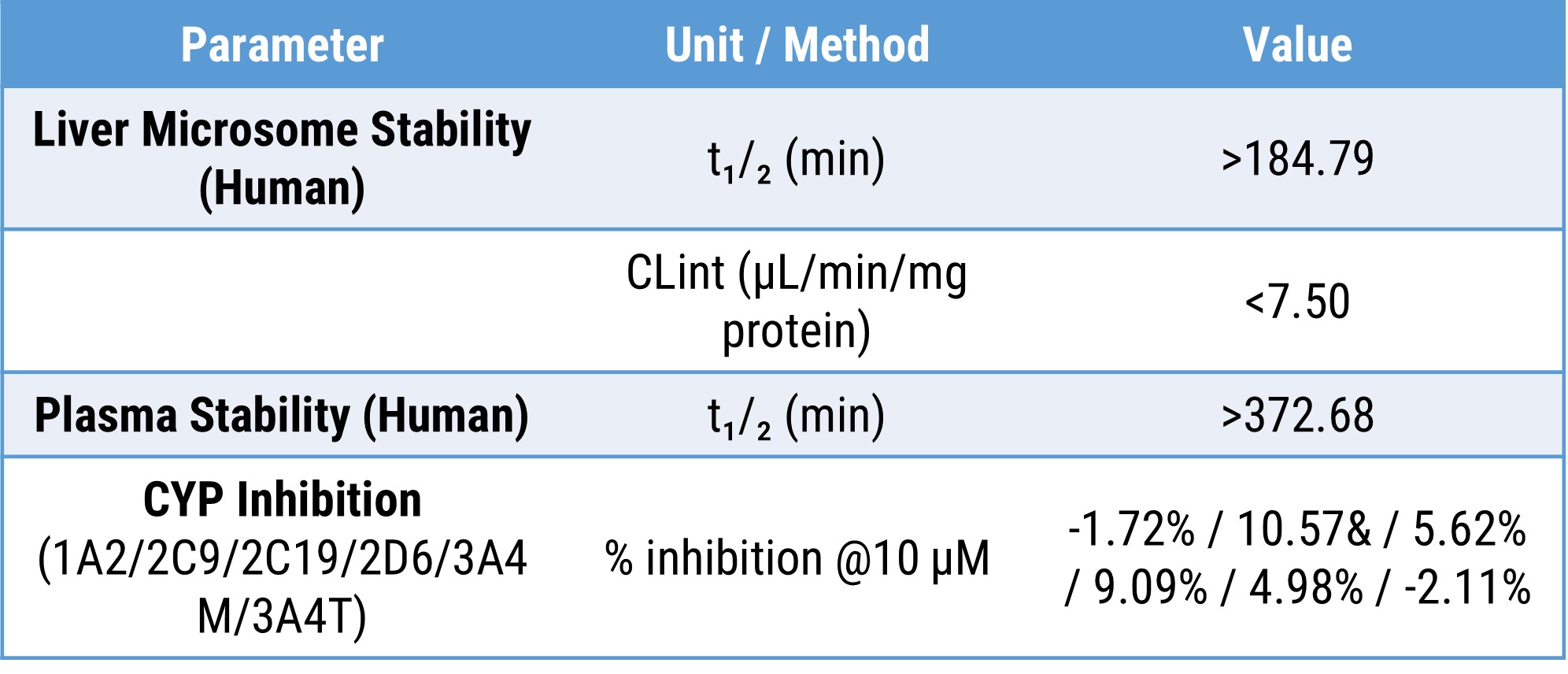
Table 1: In vitro ADME properties of the VAV1 MGD. The compound showed high metabolic stability in human liver microsomes and human plasma, with low CYP inhibition across major isoforms at 10 µM.

Table 2: Pharmacokinetic profile of the VAV1 degrader following IV and PO administration. The compound exhibits favorable oral bioavailability (F = 70.5%) with moderate clearance and comparable half-lives across both routes.
To further assess early safety risk, the compound was profiled using the ICESTP 90 SAFETYPANEL™. This broad functional panel covers 90 key targets in both agonist and antagonist modes, enabling detection of potential off-target effects.
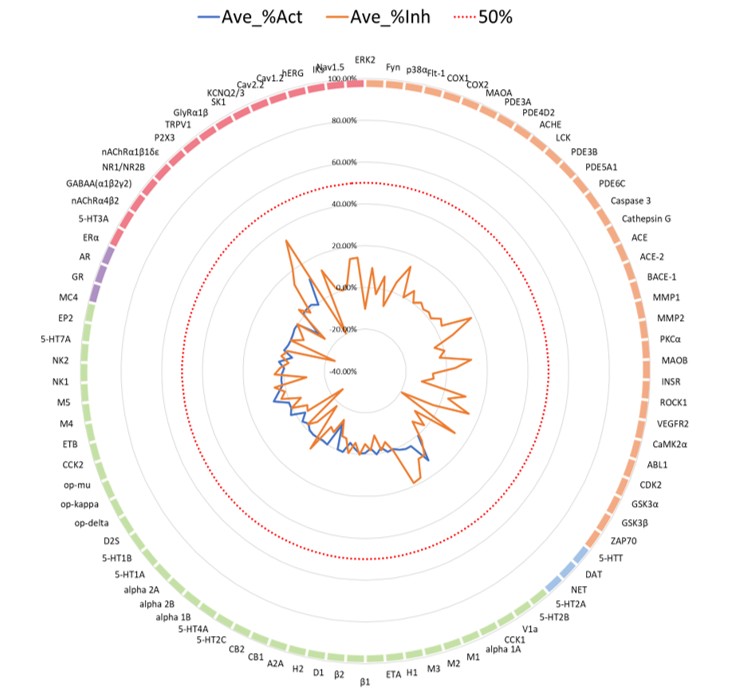
Figure 4: Safety panel profiling of the VAV1 MGD using the ICESTP 90 SAFETYPANEL™. No significant off-target activity (>50% inhibition or activation) was observed across 90 key targets, supporting a favorable early safety profile.
The assay was performed at physiological temperature (35–37 °C) in line with ICH S7B “best practice” recommendations for ion channel studies. The voltage protocol incorporated ventricular action potential features, and high-quality recordings (seal resistance, series resistance, current stability) were analyzed.
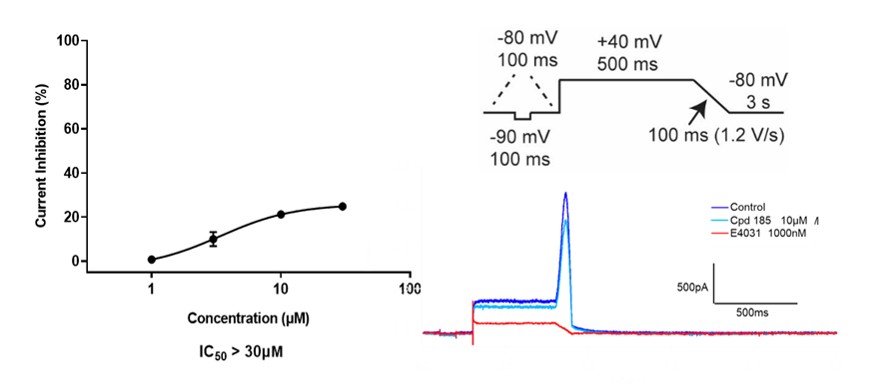
Figure 5: hERG current measured by manual patch clamp.The VAV1 MGD showed no significant inhibition of hERG current, while the positive control E4031 (1 µM) caused a marked suppression.
Through a comprehensive evaluation spanning complex formation, in vitro and in vivo degradation, mechanism of action, functional impact, and early safety profiling, we demonstrated the broad applicability of our integrated platform to support molecular glue degrader development. The VAV1-targeting MGD showed potent and selective degradation, minimal off-target effects, favorable ADME properties, and low cardiac liability. These results highlight our capability to accelerate degrader programs from discovery to preclinical decision-making.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.