Obesity and diabetes continue to rise at a global scale, now affecting over 4 billion people combined. While GLP-1 and dual GIP/GLP-1 receptor agonists have become frontline therapies, there remains strong demand for alternative mechanisms with improved efficacy, safety, and long-term adherence.
Amylin and its receptor system—comprising the calcitonin receptor (CTR) and RAMP proteins (AMY1/2/3)—have emerged as a promising target class in metabolic regulation. Amylin analogs are known to suppress appetite, delay gastric emptying, and improve glycemic control, acting synergistically with other metabolic hormones.

Figure 1. Structure and pharmacology of the human calcitonin receptor (CTR) family.
Recent clinical advances underscore the therapeutic relevance of this pathway:
- Cagrilintide, in combination with semaglutide, led to >22% body weight reduction in Phase III trials.
- Petrelintide (Zealand Pharma/Roche) and GUB-014295 (Gubra/AbbVie) highlight industry investment in long-acting, next-gen amylin receptor agonists.
- Oral agents like Amycretin are paving the way for more patient-friendly administration routes.
These developments position the Amylin/CTR axis as a high-potential target space in obesity and diabetes therapeutics—distinct yet complementary to GLP-1–based approaches.
To support drug discovery targeting the amylin and calcitonin receptor (AMY/CALCR) axis, ICE Bioscience has developed a suite of engineered cell lines overexpressing CALCR and its RAMP1/2/3 combinations (AMY1–3). These tools enable reliable and scalable assays for receptor signaling and compound screening.
GPCR-expressing cell lines were generated via lentiviral or Flip-In™ systems, followed by clone selection, gene confirmation, and functional validation using flow cytometry and HTRF-based cAMP assays.
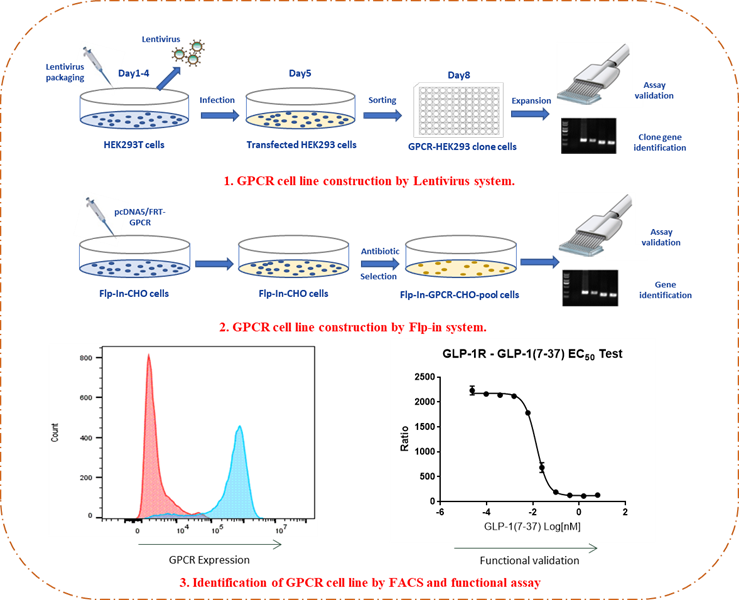
Figure 2. Workflow for establishing CALCR and AMYR-overexpressing stable cell lines.
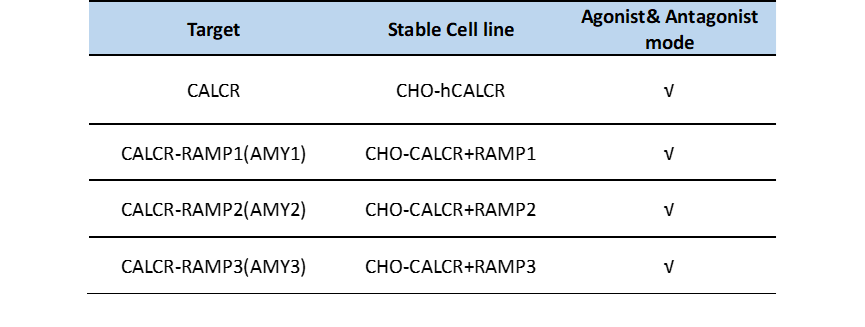
Table 1. AMY3/CTR receptor stable cell lines.
Each cell line showed high-level, specific expression of the intended RAMP isoform, confirming successful receptor subtype assembly.
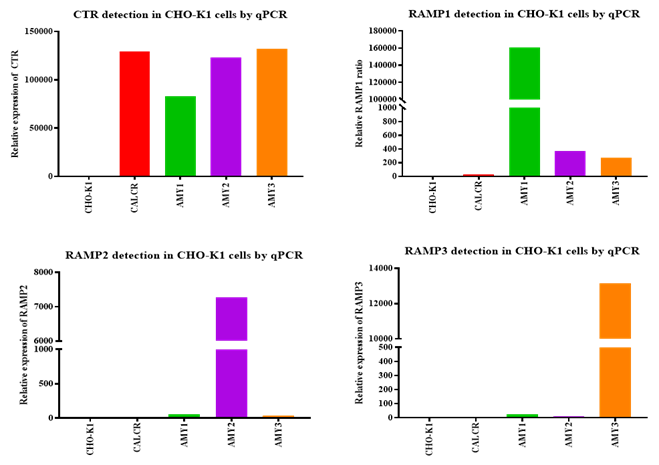
Figure 3. qPCR confirmation of RAMP expression in monoclonal CHO cell lines.
The cAMP response to hAmylin and hCT in AMY3 and CALCR-expressing cells showed expected EC₅₀ values (135 pM and 50 pM, respectively), consistent with literature-reported pharmacological activity.

Figure 4. Validation of HTRF cAMP assay using hCT and hAmylin reference ligands.
Both Petrelintide and Cagrilintide were profiled across receptor subtypes, with observed potencies and response trends matching published pharmacological data, supporting the suitability of these models for drug candidate screening.
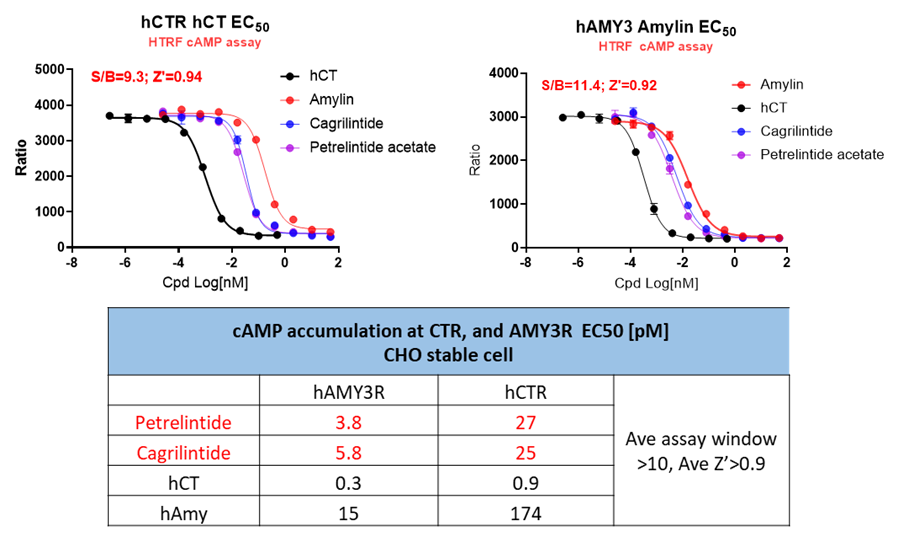
Figure 5. Pharmacological profiling of Petrelintide and Cagrilintide in hCTR and hAMY3 cell lines.
All four cell lines were configured to support both agonist and antagonist readouts, enabling flexible assay formats for diverse screening needs.
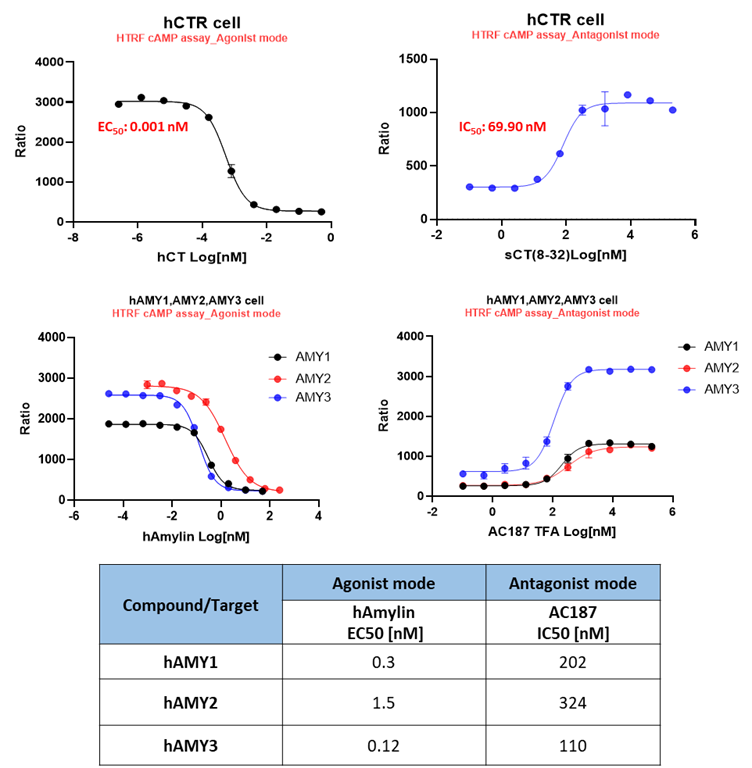
Figure 6. Assay modes established for agonist and antagonist screening.
The engineered AMY/CTR receptor cell lines developed by ICE Bioscience offers a robust and flexible platform for pharmacological characterization of ligands targeting this emerging metabolic receptor family. Combined with HTRF-based detection and validated with clinical reference compounds, this system is well-suited for HTS campaigns and mechanism-of-action studies.
2025-10-30
2025-10-23
2025-09-28
2025-08-19
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.