Selecting the right KRAS Assay Services is a pivotal step for laboratories engaged in cancer research and drug discovery. Given the critical role of KRAS mutations in approximately 95% of RAS-related oncogenic processes, accessing reliable and advanced assay platforms is essential to uncover insights into tumor biology and therapeutic targeting. A well-chosen KRAS Services Platform offers comprehensive screening, profiling, and functional assays that accelerate the evaluation of KRAS mutants, such as G12D, G12C, and Q61H, among others. This guide aims to provide a clear pathway for researchers and clinical labs to assess providers, understand the benefits of integrated KRAS assay platforms, and ensure cost-effective, accurate, and compliant services that meet evolving research demands.
Table of contents:
Critical Specifications to Evaluate in KRAS Assay Services Providers
Benefits of Utilizing a Comprehensive KRAS Services Platform for Research
Cost-Efficiency and Accuracy in RAS Assays for Clinical Labs
Ensuring Compliance and Certification with KRAS Assay Services
When selecting KRAS Assay Services, laboratories must prioritize providers that offer extensive expertise and a broad portfolio of KRAS mutant variants and related RAS family proteins. ICE Bioscience exemplifies this by providing a diverse range of recombinant proteins, including KRAS mutants (G12D/C/V/R/S, G13D/C, Q61H), HRAS, and NRAS, all validated for purity and activity. The availability of both enzymatic and biophysical assays, such as protein-protein interaction assays and Surface Plasmon Resonance (SPR) binding assays, ensures comprehensive characterization of KRAS function and inhibitor efficacy. It is also important to consider providers that support multiple assay formats, including nucleotide exchange assays and cell proliferation assays using KRAS Cancer Cell Panels and Ba/F3 cells. The inclusion of GDP-loaded proteins and the ability to assess interactions with guanine nucleotide exchange factors (SOS1, SOS2) and downstream effectors like cRAF are vital for detailed mechanistic studies. Turnaround times, assay reproducibility, and the capacity for customization further define the suitability of a KRAS services partner.
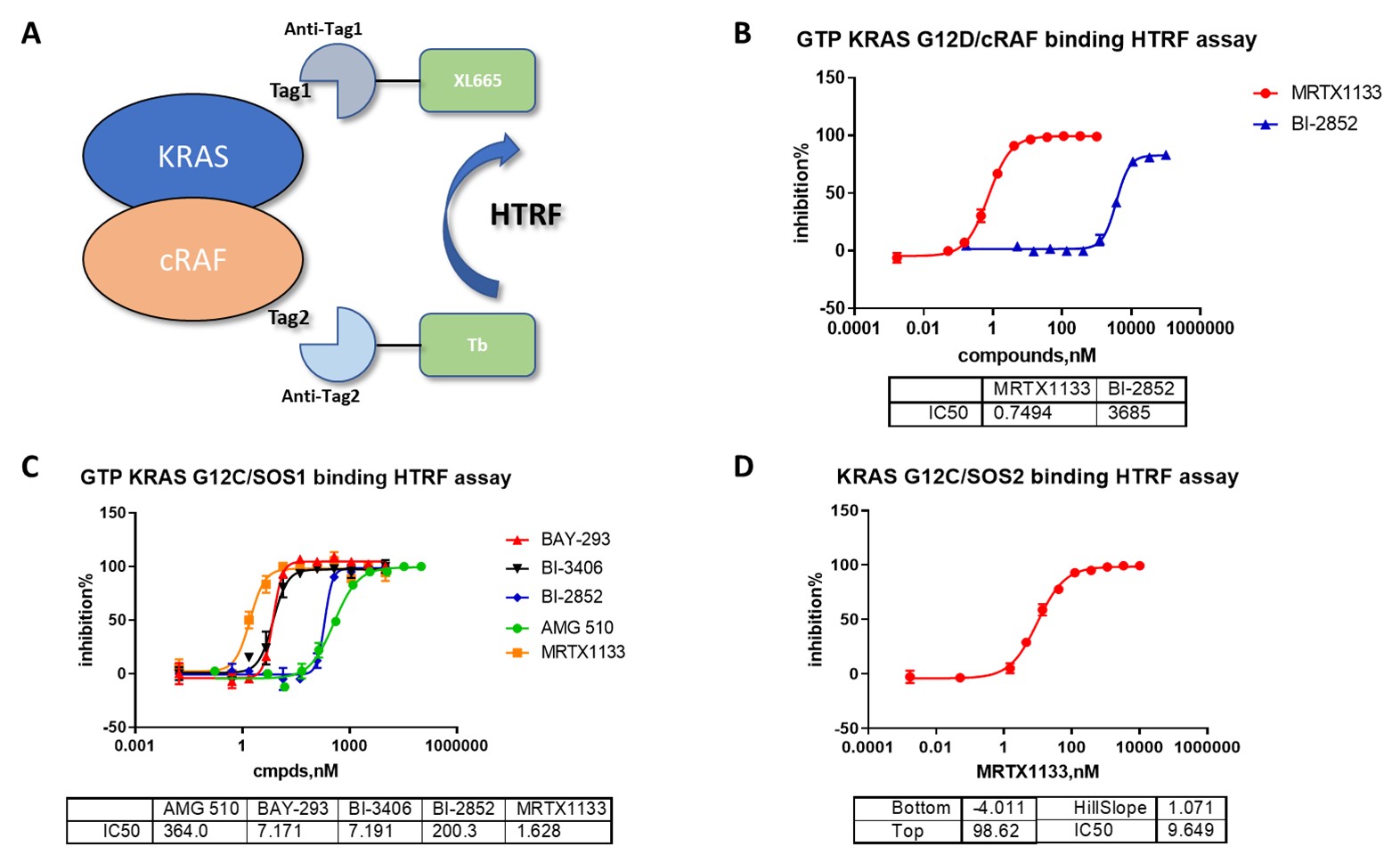
Utilizing a comprehensive KRAS Services Platform streamlines research workflows by integrating multiple assay types and mutant variants into a single, cohesive system. ICE Bioscience’s platform exemplifies this approach by combining protein purification, interaction assays, SPR binding, and cell-based proliferation studies to provide a holistic understanding of KRAS biology. This integration facilitates rapid screening of small molecule inhibitors targeting KRAS mutants, with methods such as HTRF and TR-FRET enabling sensitive detection of compound effects on nucleotide exchange activity and protein interactions. The platform’s inclusion of a wide panel of cancer cell lines, including RAS-resistant and Ba/F3 cells, allows researchers to evaluate compound efficacy across diverse genetic backgrounds and cellular contexts. Additionally, the availability of both 2D and 3D cell-based assays enhances physiological relevance. By centralizing these capabilities, researchers benefit from enhanced data consistency, reduced timelines, and improved translational potential for therapeutic development.
For clinical laboratories, balancing cost-efficiency with assay accuracy is paramount when implementing RAS assays. Providers like ICE Bioscience deliver value by offering robust KRAS Assay Services that cover a broad spectrum of KRAS mutations and related proteins, minimizing the need for multiple vendors. The use of validated recombinant proteins and standardized assay protocols ensures high reproducibility and reliable data, critical for clinical decision-making. The availability of high-throughput screening formats, such as 384-well and 96-well plates, supports scalable testing while optimizing reagent use and reducing per-sample costs. Turnaround times of approximately 2-3 weeks for cell proliferation and viability assays enable timely results without compromising quality. Furthermore, the incorporation of advanced biophysical techniques like SPR allows precise quantification of binding kinetics, enhancing the predictive power of assays. Such comprehensive yet cost-effective platforms empower clinical labs to deliver accurate RAS mutation profiling and drug response evaluation efficiently.
Compliance with regulatory standards and certification requirements is a critical consideration when procuring KRAS Assay Services. Laboratories must select providers that adhere to stringent quality control measures, including batch-to-batch validation of recombinant proteins for purity and biological activity. ICE Bioscience maintains rigorous activity validation for all KRAS mutant proteins and related assay reagents, ensuring consistency and reliability. Additionally, the use of well-established assay methodologies, such as HTRF and TR-FRET, supports standardized data generation compatible with regulatory expectations. Providers should also offer detailed documentation, including datasheets and assay protocols, to facilitate audit readiness and traceability. The ability to customize assays while maintaining compliance with industry standards enhances flexibility without sacrificing quality. Ultimately, partnering with a KRAS services provider committed to certification and quality assurance safeguards the integrity of research and clinical outcomes.
In summary, selecting the ideal KRAS Assay Services and RAS assays involves careful evaluation of provider capabilities, assay comprehensiveness, cost-efficiency, and regulatory compliance. ICE Bioscience’s KRAS Services Platform exemplifies a robust solution that addresses these criteria through a wide array of validated KRAS mutant proteins, integrated biochemical and cellular assays, and advanced biophysical techniques. Their offerings enable researchers and clinical labs to conduct detailed mechanistic studies, screen potential inhibitors, and profile cancer cell responses efficiently. By leveraging such a comprehensive platform, laboratories can accelerate drug discovery and improve the accuracy of KRAS mutation analyses, ultimately contributing to more effective cancer therapeutics development. Choosing a trusted partner with proven expertise and commitment to quality ensures that your lab’s KRAS research and clinical testing needs are met with precision and reliability.
2026-02-04
2026-02-04
2026-02-04
2026-02-04
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.