In the evolving landscape of cancer research and drug discovery, the KRAS Services Platform has emerged as a pivotal resource for understanding oncogenic mutations and accelerating therapeutic development. KRAS mutations, which constitute approximately 95% of RAS gene alterations in human cancers, present a complex challenge due to their role in driving uncontrolled cell proliferation and tumor progression. Outsourcing KRAS assay services offers laboratories and pharmaceutical companies access to advanced technologies and expert resources without the need for extensive in-house infrastructure. This strategic approach not only enhances research capabilities but also optimizes operational efficiency, enabling faster and more reliable results. By leveraging specialized KRAS assay services, organizations can focus on core competencies while benefiting from cutting-edge assays that support precision oncology and targeted therapy development.
Table of contents:
How Outsourced KRAS Services Platform Enhances Laboratory Scalability
Risk Mitigation and Quality Assurance in KRAS Assay Services Partnerships
Cost-Benefit Analysis of External RAS Assays versus In-House Testing
Selecting Reliable KRAS Services Platform Providers for Long-Term Success
Outsourcing to a dedicated KRAS Services Platform significantly enhances laboratory scalability by providing access to a broad spectrum of assays and mutant variants without the constraints of internal resource limitations. Platforms like ICE Bioscience offer comprehensive KRAS screening and profiling services, including protein purification, protein-protein interaction assays, and surface plasmon resonance (SPR) binding assays with various KRAS mutants such as G12D, G12C, G12V, and others. These services allow laboratories to scale their testing volume efficiently, accommodating fluctuating project demands. Additionally, the availability of specialized cell panels, including KRAS cancer cell and Ba/F3 cell proliferation assays, supports diverse experimental needs ranging from 2D and 3D cell-based assays to high-throughput screening formats. By outsourcing, laboratories can bypass the lengthy setup times and high capital investments associated with developing these complex assays internally, thus accelerating drug discovery timelines and expanding research capacity without compromising quality.
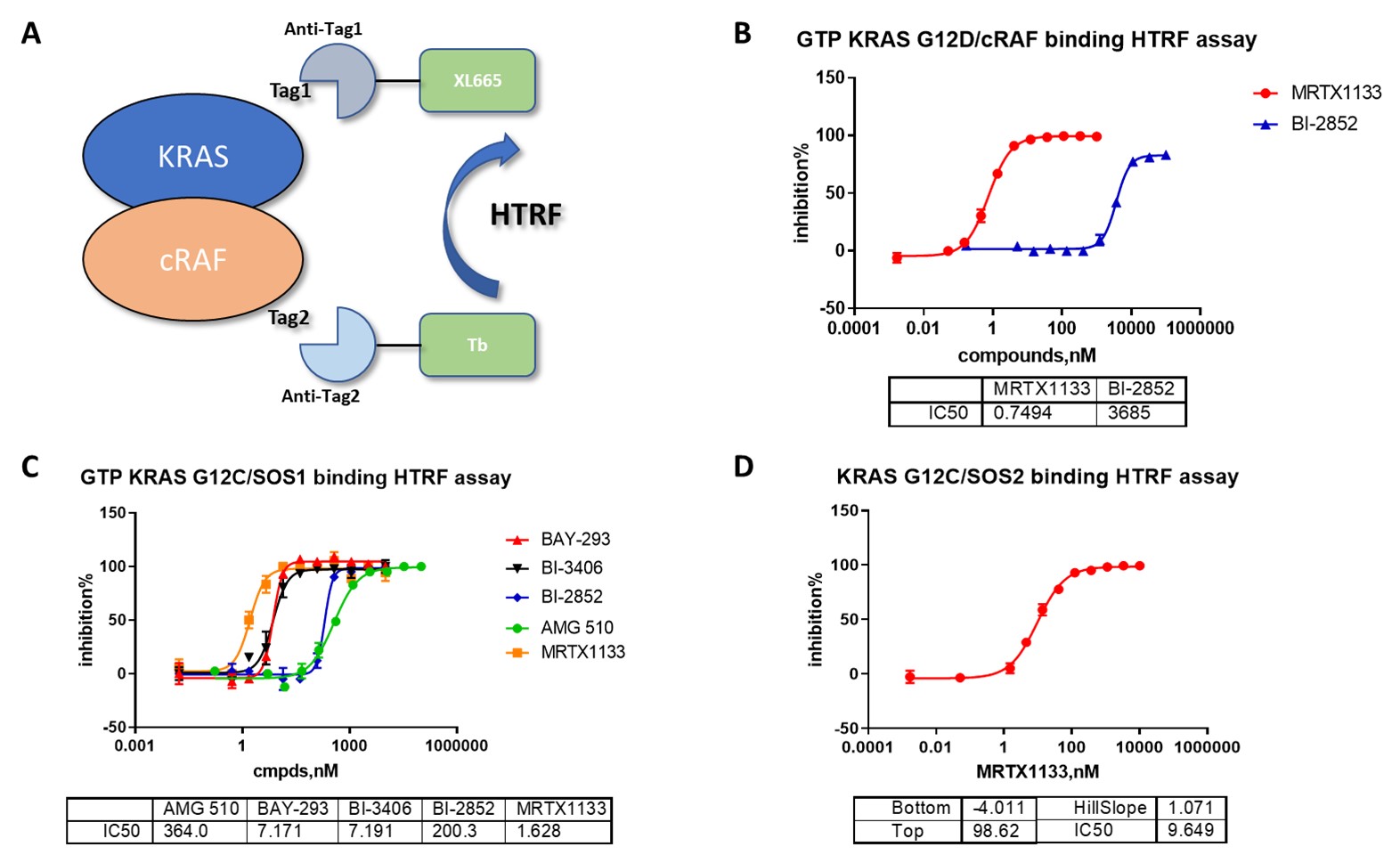
Partnering with established providers of KRAS Assay Services ensures stringent risk mitigation and superior quality assurance, critical factors in sensitive oncological research. Providers like ICE Bioscience implement rigorous validation protocols for each batch of recombinant proteins, including KRAS mutants and related proteins such as HRAS, NRAS, SOS1, and cRAF. These proteins undergo thorough activity validation to guarantee purity and functionality, reducing experimental variability and enhancing reproducibility. Moreover, advanced assay platforms such as HTRF and TR-FRET are employed to monitor protein interactions and nucleotide exchange activities with high sensitivity and specificity. Outsourcing also minimizes operational risks associated with technical errors, equipment failures, and regulatory compliance challenges that often burden in-house laboratories. By entrusting KRAS assay execution to experts, organizations benefit from consistent assay performance, validated data, and adherence to best practices, which collectively strengthen the reliability of research findings and support regulatory submissions.
Conducting a cost-benefit analysis reveals that outsourcing RAS assays, particularly through a specialized KRAS Services Platform, often offers superior financial and operational advantages compared to in-house testing. Establishing and maintaining in-house KRAS assay capabilities requires substantial investment in sophisticated instrumentation, skilled personnel, and continuous quality control measures. Additionally, the complexity of KRAS biology and the need for multiple mutant variants and assay formats increase operational costs. External service providers amortize these costs across numerous clients, enabling access to state-of-the-art technologies at a fraction of the expense. Furthermore, outsourcing reduces overhead related to reagent procurement, assay development, and troubleshooting. The rapid turnaround times offered by platforms like ICE Bioscience—typically two to three weeks—also translate into time savings, accelerating project milestones and reducing time-to-market. Ultimately, outsourcing KRAS assay services provides a cost-effective, scalable, and high-quality solution that supports efficient resource allocation and strategic growth.
Choosing a reliable KRAS Services Platform provider is essential for ensuring long-term success in cancer research and drug development. Key criteria include the provider’s technical expertise, assay portfolio breadth, quality control standards, and customer support responsiveness. ICE Bioscience exemplifies these qualities by offering a wide range of KRAS mutant proteins, validated biochemical and cellular assays, and innovative technologies such as SPR and HTRF. Their comprehensive service portfolio encompasses enzymatic, biophysical, and cellular assays designed to facilitate drug discovery and expedite development processes. Moreover, providers with extensive experience in KRAS biology and proven track records in delivering reproducible data can better support customized assay development and complex study designs. Transparent communication, flexible assay formats, and timely delivery further contribute to a successful partnership. Selecting a trusted KRAS assay services partner enables organizations to navigate the intricacies of RAS-driven cancers with confidence, fostering innovation and sustained research excellence.
In summary, outsourcing KRAS assay services through a specialized platform offers strategic benefits including enhanced scalability, risk mitigation, cost efficiency, and access to expert-driven quality assurance. Platforms such as those provided by ICE Bioscience deliver comprehensive solutions encompassing mutant protein production, interaction assays, and cellular proliferation studies that are indispensable for advancing oncology research. By leveraging outsourced KRAS services, laboratories and pharmaceutical companies can optimize their operational workflows, reduce financial burdens, and accelerate therapeutic discovery. Careful selection of a reliable KRAS services provider ensures sustained success and positions organizations at the forefront of precision medicine targeting RAS-driven malignancies. This strategic approach ultimately empowers researchers to focus on innovation while relying on robust, validated assay platforms.
.
2026-02-04
2026-02-04
2026-02-04
2026-02-04
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.