Isothermal Titration Calorimetry (ITC) is a biophysical technique for directly measuring the thermodynamics of molecular interactions. By monitoring the heat released or absorbed during a binding event, ITC provides a comprehensive profile of the interaction, including binding affinity (Kd), stoichiometry (n), enthalpy (ΔH), and entropy (ΔS). This label-free and solution-based method is ideal for studying a wide range of biomolecular interactions, such as protein-ligand, protein-protein, and nucleic acid interactions. ITC enables detailed characterization of binding mechanisms, making it an invaluable tool for drug discovery, lead optimization, and biomolecular research.
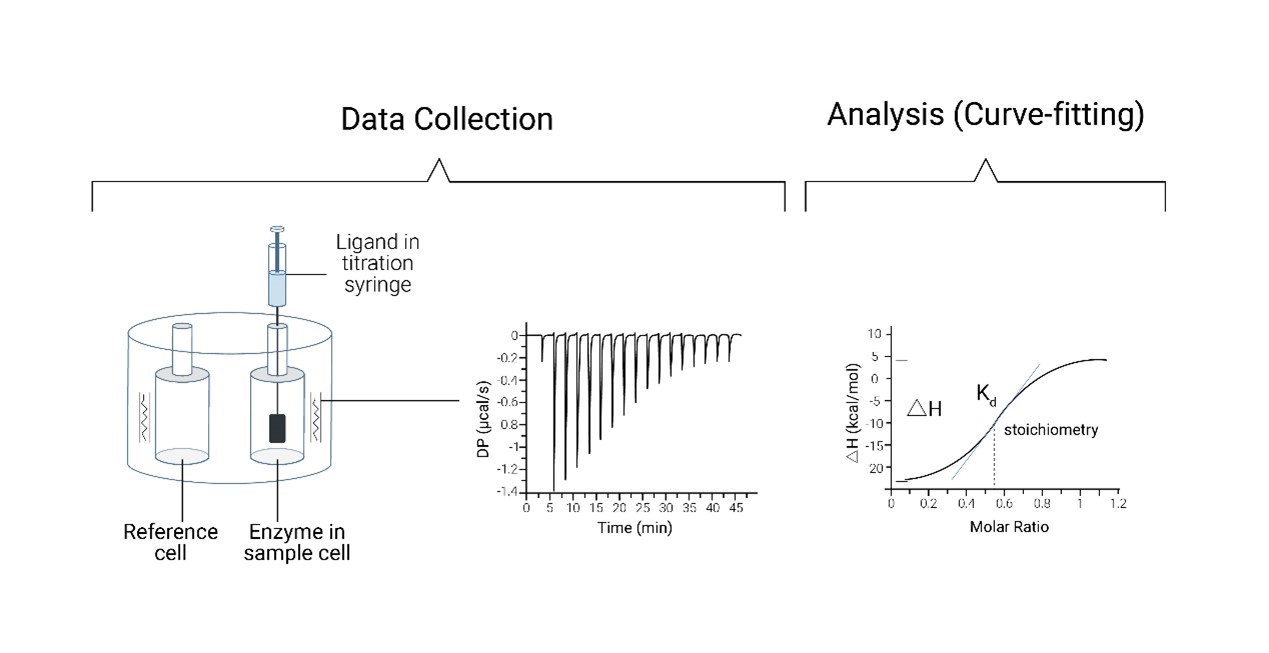
In an ITC assay, a solution containing the ligand is gradually titrated into a solution containing the target molecule under isothermal (constant temperature) conditions. The heat released or absorbed during each binding event is measured with high sensitivity, allowing for the calculation of key thermodynamic parameters, including binding affinity (Kd), stoichiometry (n), enthalpy (ΔH), and entropy (ΔS).
In-House Proteins: Our highly active, in-house produced proteins enable a wide range of biophysical assays, ensuring high precision, reliability, and reproducibility.
Versatile Applications: Label-Free and Native Conditions; Physiologically Relevant; Robust with Challenging Samples – Works with turbid or colored solutions; optical clarity not required; Comprehensive Thermodynamic Data – One experiment yields K_d, ΔH, ΔS, ΔG, and binding stoichiometry.
Broad Range of Biomolecules: Ideal for studying a variety of biomolecular interactions, including protein-protein, protein-small molecule, and enzyme-ligand interactions.
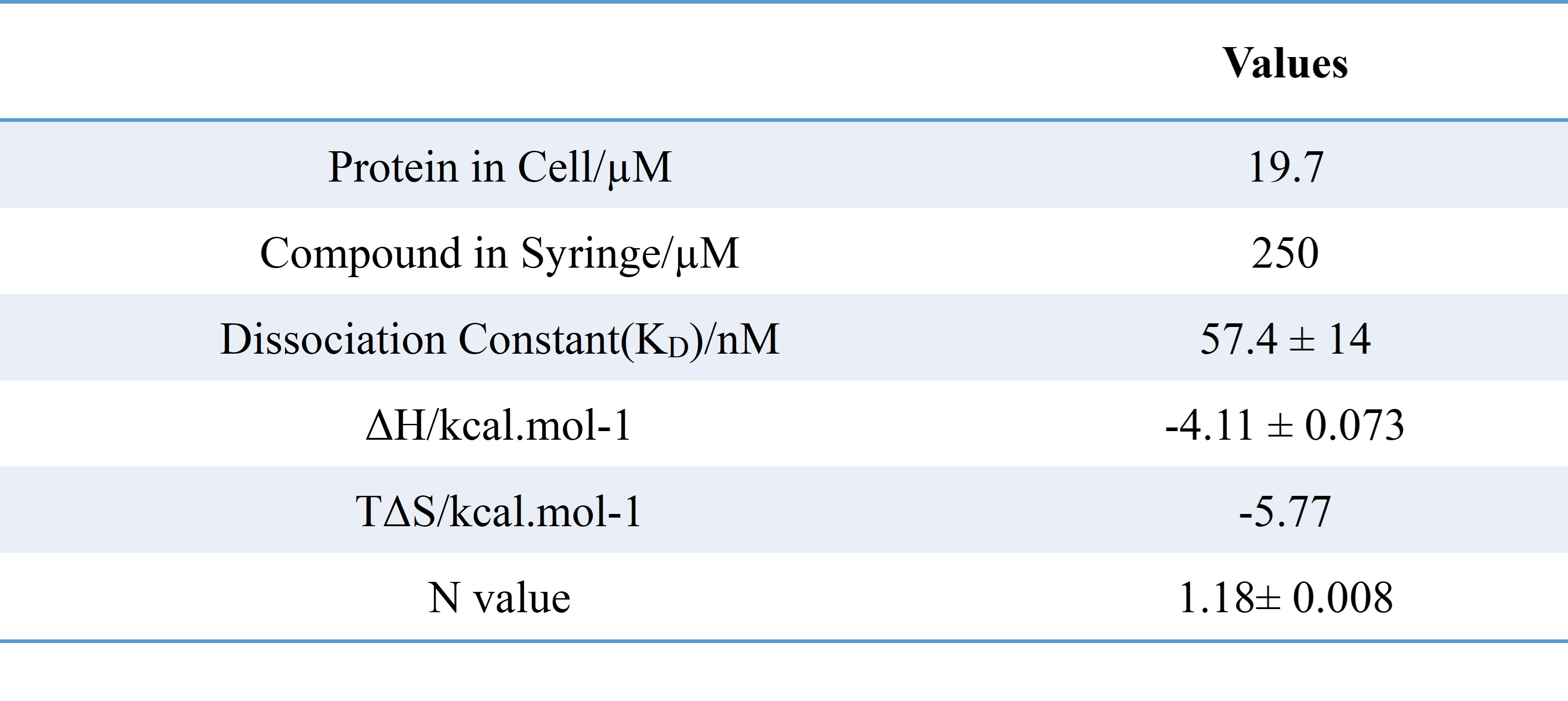
AK-1690, a STAT6 SH2 domain targeted PROTAC (Wang et al. 2024) was titrated to the non-phosphorylated STAT6 core domain in PEAQITCTM. The ITC data was analyzed using the ‘One Set of Sites’ model. The binding affinity determined from ITC was comparable to that from SPR (KD = 33.7 nM). The binding event was driven by both the enthalpy (ΔH) and entropy (TΔS) changes. The ITC ‘N value’ (1.18) confirmed the 1:1 molar ratio of STAT6:AK-1690 binding.
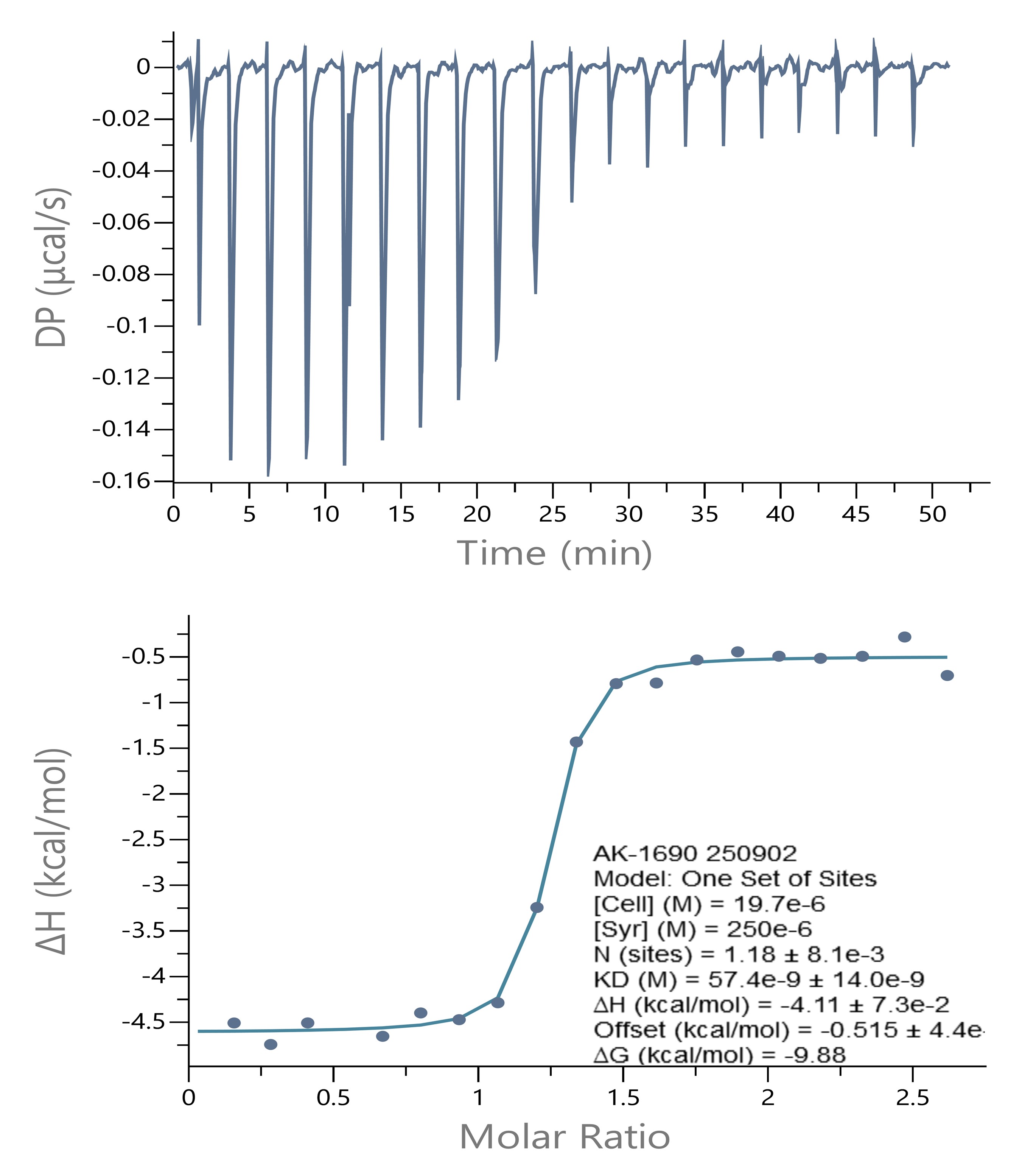
ITC Binding Isotherm of AK-1690 to STAT6.
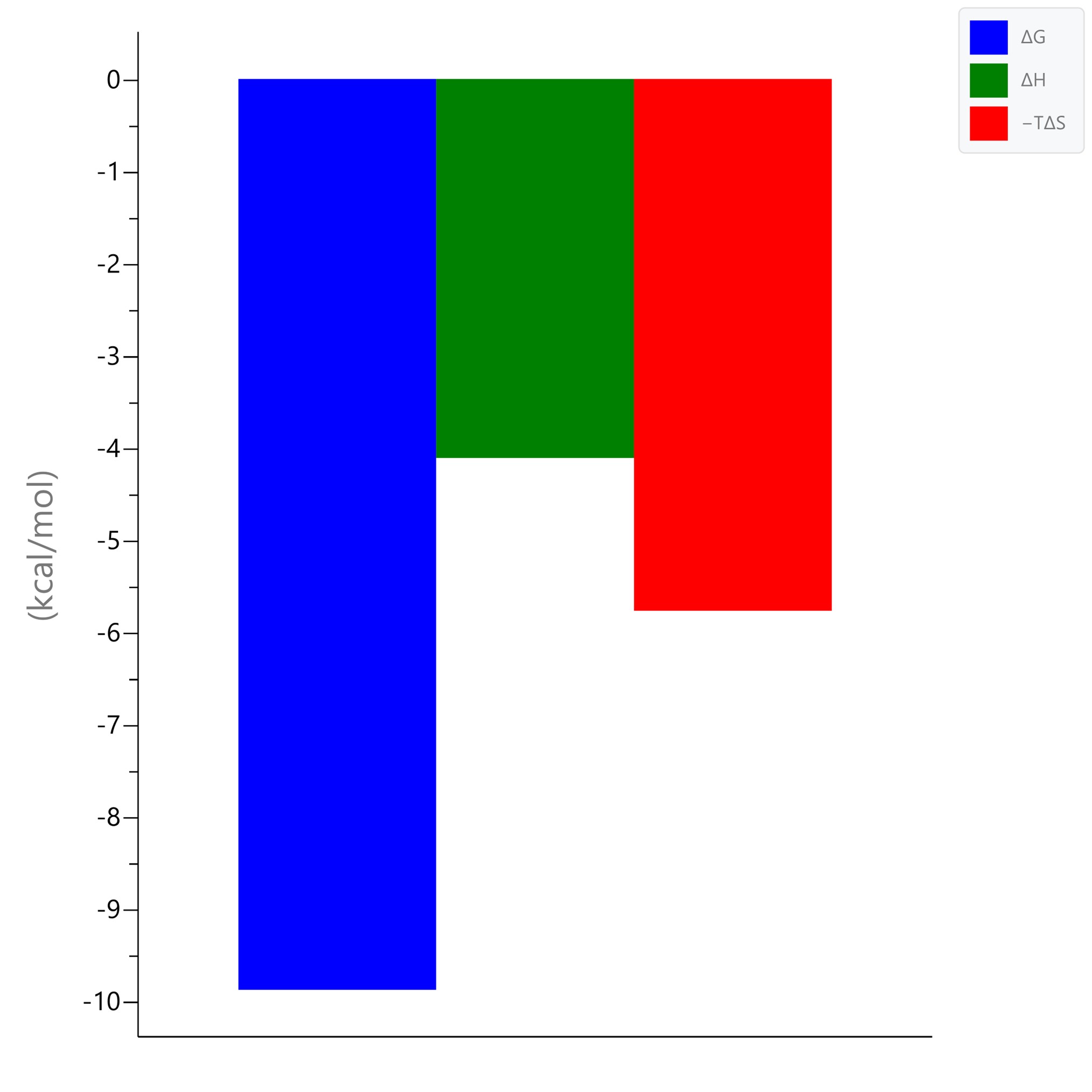
AK-1690 vs. STAT6 of binding energetics.
ITC-derived binding parameters for the AK-1690–STAT6 interaction are summarized below:
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.