Degrader-Antibody Conjugates (DACs) are an emerging therapeutic modality that combine antibody-based targeting with intracellular protein degradation. By linking a tumor-targeting antibody to a small molecule degrader, DACs enable selective degradation of intracellular targets in specific cell types, offering a new strategy for difficult-to-drug proteins.
ORM-5029 is a HER2-targeted DAC delivering a GSPT1 degrader, designed to induce selective cytotoxicity in HER2-expressing cancer cells. As a representative molecule, ORM-5029 integrates key features of the DAC platform: tumor-specific delivery, molecular glue–mediated degradation, and potent anti-tumor activity.
To evaluate the intracellular degradation efficiency of ORM-5029, we conducted GSPT1 quantification assays in BT-474 cells, a HER2-positive human breast cancer cell line.
Using both flow cytometry (FC) and Western blotting (WB), ORM-5029 treatment resulted in a clear and concentration-dependent reduction of GSPT1 protein levels. As shown in the FC histogram, GSPT1 signal intensity was significantly reduced after treatment with ORM-5029, in a pattern comparable to benchmark degraders such as CC-885, CC-90009, and SMol006.
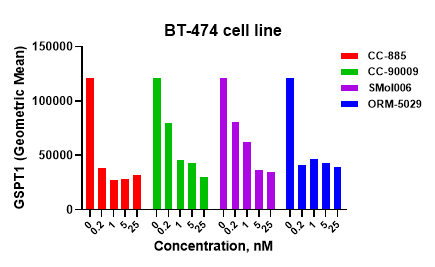
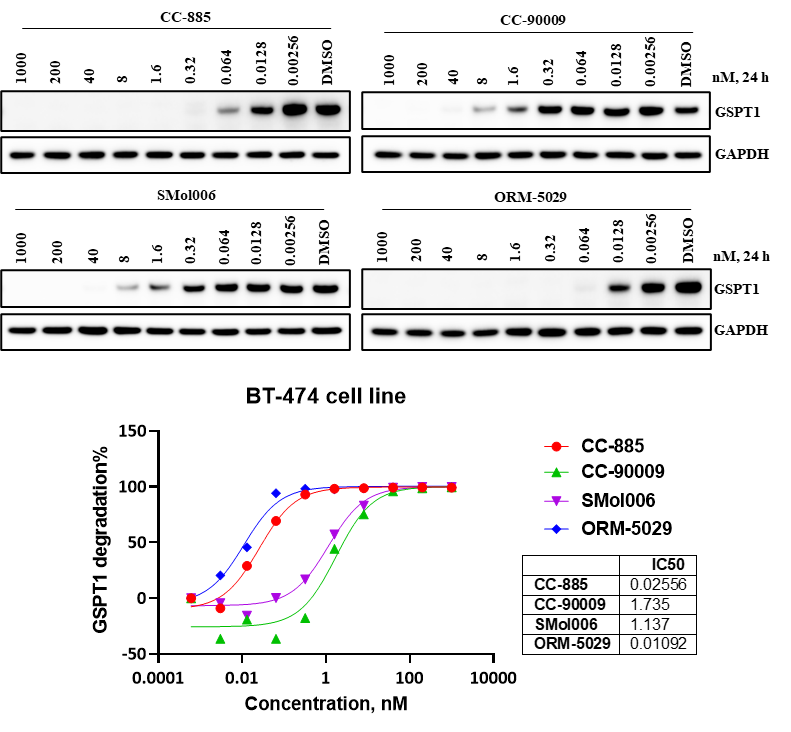
Western blot analysis confirmed the efficient depletion of GSPT1 protein following exposure to ORM-5029. The dose-response curve showed an IC₅₀ of 0.01092 nM, outperforming all reference GSPT1 degraders tested, including CC-885 (0.02556 nM), indicating highly potent degradation activity.
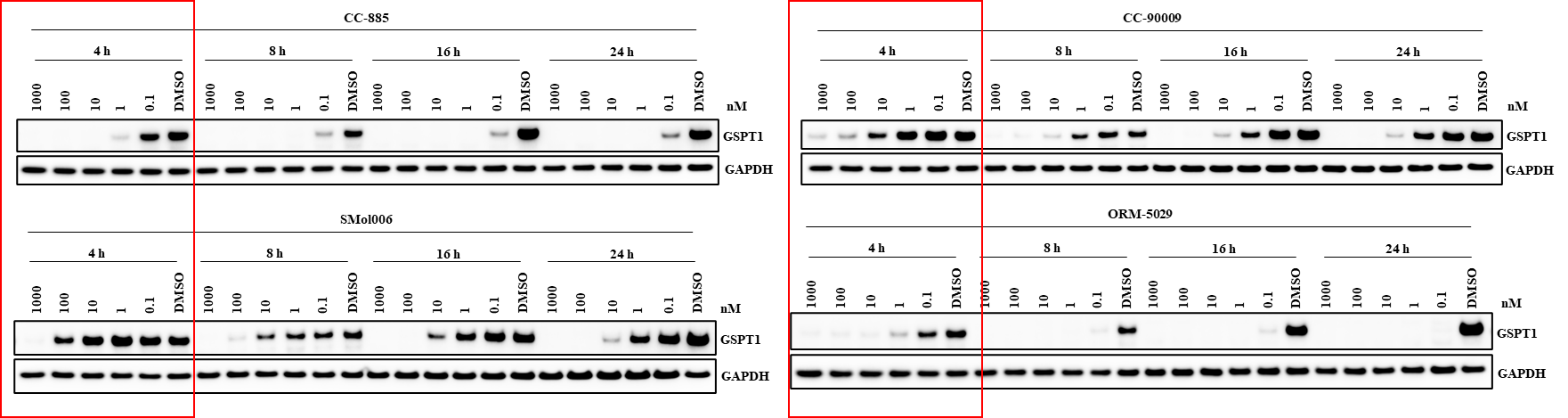
We offer time-course Western blot analysis to evaluate DAC-induced protein degradation kinetics. In HER2+ BT-474 cells, ORM-5029 treatment led to rapid and sustained GSPT1 degradation. This assay enables comparison of degrader onset, duration, and potency to support candidate optimization.
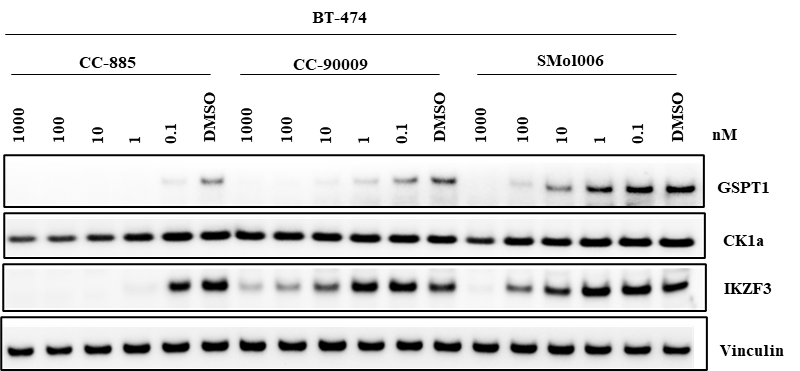
Understanding the degrader selectivity is essential to optimizing the therapeutic index of DACs. Even when antibody targeting is precise, off-target protein degradation within target cells can impact safety and efficacy.
We provide off-target profiling services for DAC payloads by detecting degradation of known neo-substrates using Western blot. In this study, the GSPT1 degrader payload SMol006 was evaluated in HER2+ BT-474 cells. In addition to on-target GSPT1 loss, partial degradation of other CRBN-recruited proteins was observed, indicating off-target activity.
To demonstrate functional efficacy of DAC molecules, we provide target-dependent cell killing assays across tumor cell lines with defined antigen expression. This setup enables evaluation of both payload potency and antibody-mediated delivery efficiency. By comparing DAC constructs, free degraders, and non-targeted ADCs, we assess the contribution of each component to cytotoxic activity.
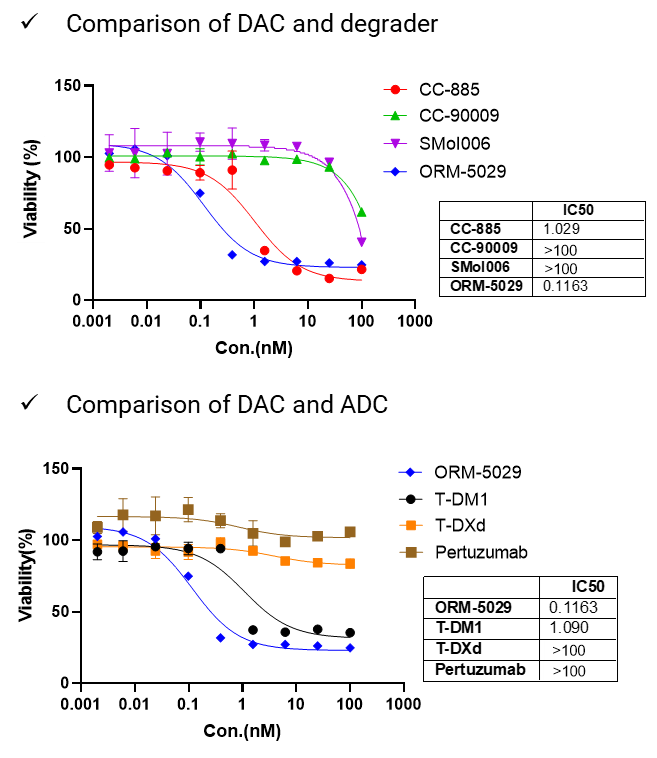
To evaluate the target dependency of DAC activity, we offer cell-based cytotoxicity assays across tumor models with varying antigen expression. Clear correlation was observed between HER2 expression and cytotoxic response, highlighting the requirement for antibody-mediated delivery.
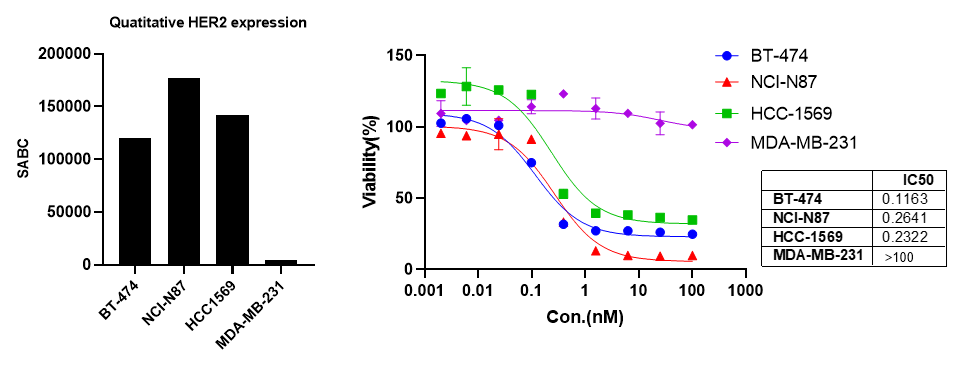
We provide integrated in vitro and in vivo assay platforms to evaluate the efficacy of DAC candidates. Using the HER2+ HCC1569 breast cancer model, ORM-5029 demonstrated potent cytotoxicity in vitro and robust tumor growth inhibition in a cell-derived xenograft (CDX) model. Body weight remained stable throughout treatment, indicating good tolerability.
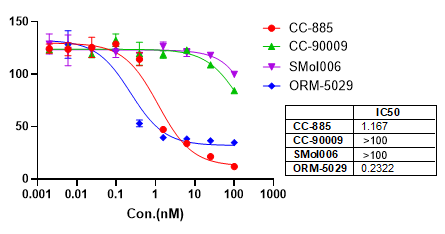
In vitro cell killing assay.
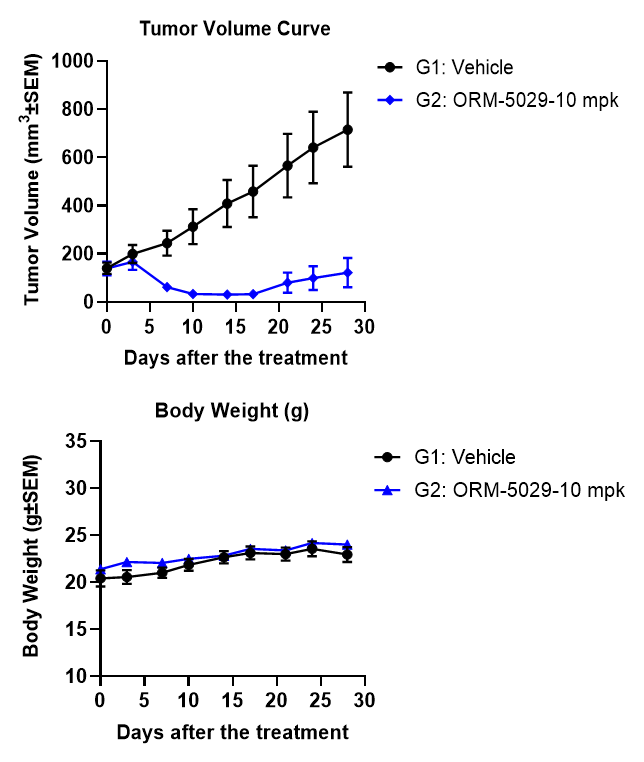
HCC1569 CDX Model.
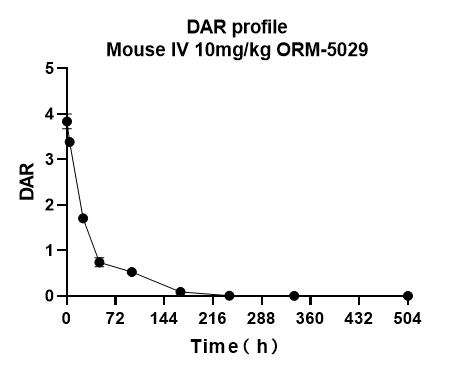
We offer pharmacokinetic and drug-to-antibody ratio (DAR) studies to support preclinical DAC development.
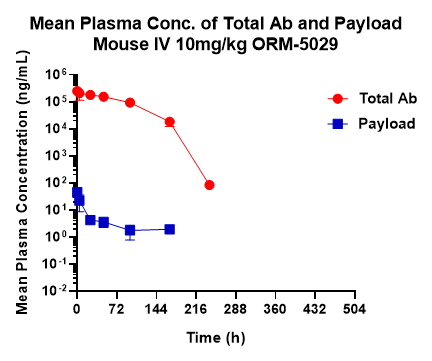
ORM-5029 showed a half-life of about 34 hrs based on total antibody measurements.
Our bioanalytical workflow enables monitoring of total antibody, payload, and DAR to characterize in vivo stability and release kinetics of DACs.
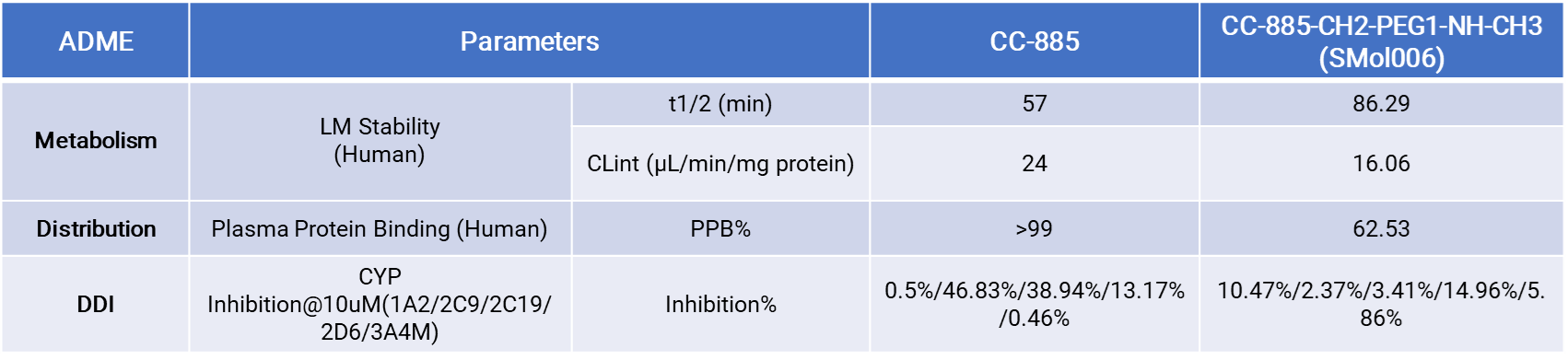
We provide comprehensive ADME profiling to support payload selection and optimization for DAC development. Key assays include liver microsome stability, intrinsic clearance (CLint), plasma protein binding (PPB%), and CYP inhibition panels for drug–drug interaction (DDI) risk assessment.
Our integrated DAC platform enables comprehensive evaluation of degrader–antibody conjugates from early discovery through translational development. By combining mechanistic insights with rigorous bioanalytical and pharmacological testing, our platform provides a powerful framework to optimize DAC design, assess safety and efficacy, and de-risk clinical progression. These end-to-end solutions are designed to help our partners accelerate the development of next-generation targeted protein degradation therapeutics.
2025-10-30
2025-10-23
2025-09-28
2025-08-15
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.