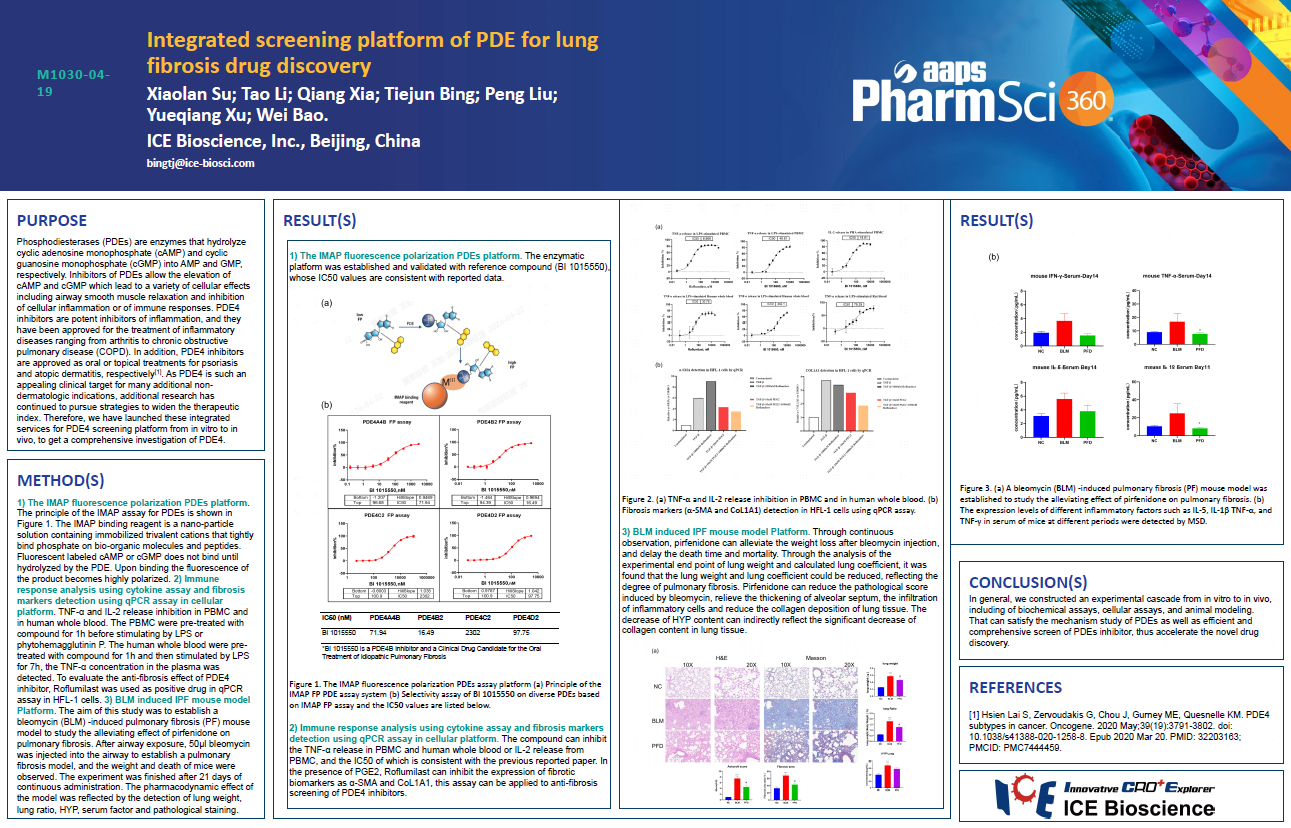
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) into AMP and GMP, respectively. Inhibitors of PDEs allow the elevation of cAMP and cGMP which lead to a variety of cellular effects including airway smooth muscle relaxation and inhibition of cellular inflammation or of immune responses. PDE4 inhibitors are potent inhibitors of inflammation, and they have been approved for the treatment of inflammatory diseases ranging from arthritis to chronic obstructive pulmonary disease (COPD). In addition, PDE4 inhibitors are approved as oral or topical treatments for psoriasis and atopic dermatitis, respectively. As PDE4 is such an appealing clinical target for many additional non-dermatologic indications, additional research has continued to pursue strategies to widen the therapeutic index. Therefore, we have launched these integrated services for PDE4 screening platform from in vitro to in vivo, to get a comprehensive investigation of PDE4.

DNA damage represents a critical threat to cellular viability, as improperly repaired DNA damage can lead to cellular senescence, apoptosis, or tumorigenesis. The DNA damage response (DDR) encompasses a series of cellular processes that detect and repair genomic lesions. Targeting DDR pathways and inhibiting DNA repair mechanisms have emerged as promising strategies in cancer therapy, and significant progress has been made in the discovery of DDR-related inhibitors. However ,drug resistance has become an increasingly prevalent challenge in this field. To better understand compound potency across various cancer cell lines, we generated drug-sensitive and drug-resistant cell lines by knocking out key DDR genes (e.g., BRCA1/2, XRCC1) and culturing cells under selective pressure from different DDR-targeting compounds. Additionally, together with wild-type (WT) cells commonly used in DDR-related drug discovery, we established a DDR cell panel encompassing 14 distinct cancer types. This panel has been rigorously validated through in vitro proliferation assays and in vivo efficacy studies. Further more, sequencing and bioinformatic analyses have been employed to elucidate the mechanisms underlying drug sensitivity and resistance. Our findings demonstrate that this DDR cell panel offers a rapid and comprehensive platform for evaluating DDR inhibitors, thereby facilitating the efficient discovery of novel therapeutics in cancer treatment.
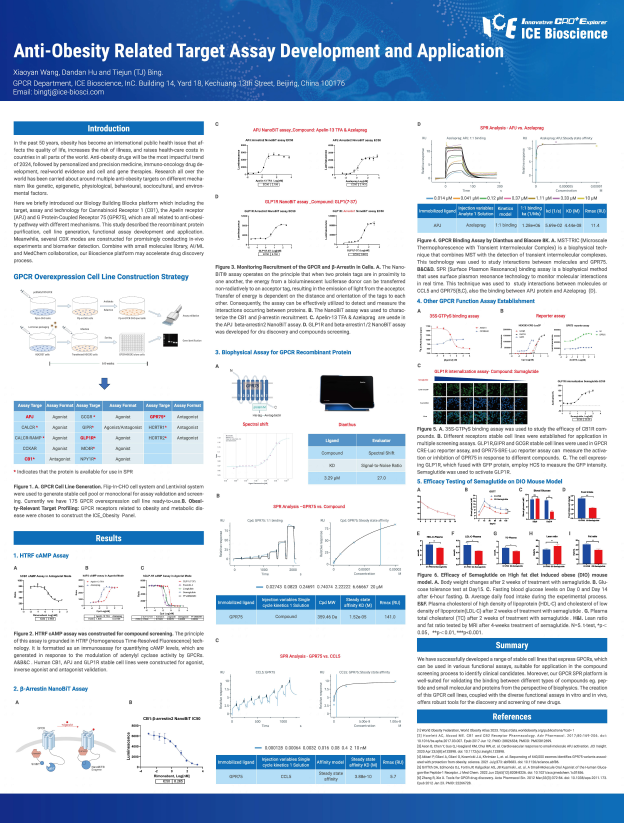
In the past 50 years, obesity has become an international public health issue that affects the quality of life, increases the risk of illness, and raises health-care costs in countries in all parts of the world. Anti-obesity drugs will be the most impactful trend of 2024, followed by personalized and precision medicine, immuno-oncology drug development, real-world evidence and cell and gene therapies. Research all over the world has been carried about around multiple anti-obesity targets on different mechanism like genetic, epigenetic, physiological, behavioural, sociocultural, and environmental factors.
Here we briefly introduced our Biology Building Blocks platform which including the target, assay and technology for Cannabinoid Receptor 1 (CB1), the Apelin receptor (APJ) and G Protein-Coupled Receptor 75 (GPR75), which are all related to anti-obesity pathway with different mechanisms. This study described the recombinant protein purification, cell line generation, functional assay development and application. Meanwhile, several CDX modes are constructed for promisingly conducting in-vivo experiments and biomarker detection. Combine with small molecules library, Al/ML and MedChem collaboration, our Bioscience platform may accelerate drug discovery process.

WRN is a nuclear protein with two known enzymatic functions: a 3'-5' exonuclease activity residing in the amino-terminal region and ATP-dependent 3'-5' DNA helicase unwinding activity, which has roles in various cellular processes that are crucial for the maintenance of genome stability.
Cancers with microsatellite instability (MSI) result from mutations in mismatch repair genes, leading to broadly distributed insertions and/or deletions of TA-dinucleotide repeats within microsatellite regions across the genome.
WRN is required for unwinding problematic DNA structures found in MSI-H cells, and the loss of WRN during cell replication leads to dsDNA breaks and the initiation of a DNA repair response. WRN emerges as a promising synthetic lethal target in MSI-H cancers.