
DNA damage is a major threat to cell survival. Not properly repaired DNA damage can lead to cell senescence, apoptosis or tumor development. DDR (DNA Damage Repair) is a collection of bioprocesses which cells utilize in order to identify and correct the DNA damage of the genome. The usage of various compounds for targeting DNA damage responses as well as attenuating DNA repair is also an approach for cancer therapy that has been widely studied in recent years. Despite the success of drug discovery of DDR related inhibitor, drug resistance has become an emerging issue of the field. Here we have constructed multiple cancer cell lines which are either DDR gene, such as XRCC1 and FANCD2, knock-out or resistant to certain DDR inhibitors. Together with the corresponding parental cell lines, we have generated a DDR cell panel which covers 12 different cancer types. We have first validated those DDR inhibitor sensitive or resistant cell lines with functional cell proliferation assays. Additionally, RNAseq has been performed for all constructed resistant cell lines. In combination with a thorough bioinformatic analysis using our in-house generated algorithms, we can provide the genetic background information of the resistant cell lines and top features that potentially contribute to the resistance mechanism. The representative data in this study has shown that LINC02709, BDKRB1, and PRKCQ are the top featured genes in A375 Vemurafenib resistant cell. Meanwhile, cell cycle, ECM-receptor interaction, and DNA replication are the most enriched KEGG terms. Lastly, we have validated this cell panel against multiple inhibitors targeting different DDR vital proteins such as ATM, PARP, POLQ etc. The result has shown that our unique DDR cell panel is capable of providing fast and comprehensive evaluation of DDR related inhibitors, thus facilitate faster and more efficient discovery of DDR inhibitors in the cancer therapy field.
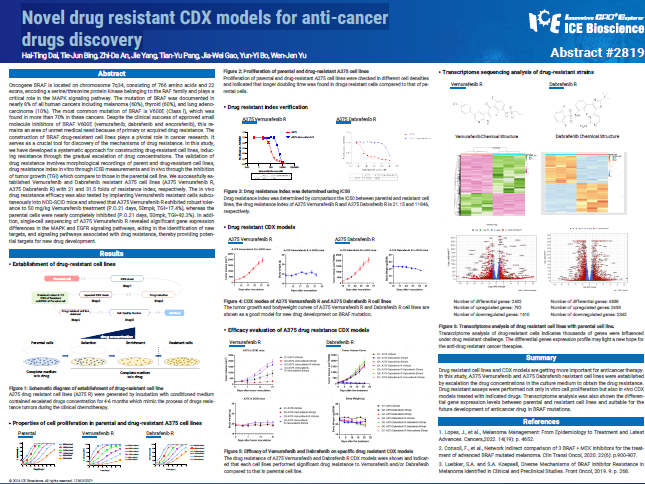
Oncogene BRAF is located on chromosome 7q34, consisting of 766 amino acids and 22 exons, encoding a serine/threonine protein kinase belonging to the RAF family and plays a critical role in the MAPK signaling pathway. The mutation of BRAF was documented in nearly 8% of all human cancers including melanoma (60%), thyroid (60%), and lung adenocarcinoma (10%). The most common mutation of BRAF is V600E (Class l), which was found in more than 70% in these cancers. Despite the clinical success of approved small molecule inhibitors of BRAF V600E (vemurafenib, dabrafenib and encorafenib), this remains an area of unmet medical need because of primary or acquired drug resistance. The construction of BRAF drug-resistant cell lines plays a pivotal role in cancer research. It serves as a crucial tool for discovery of the mechanisms of drug resistance. In this study, we have developed a systematic approach for constructing drug-resistant cell lines, inducing resistance through the gradual escalation of drug concentrations. The validation of drug resistance involves morphological recordings of parent and drug-resistant cell lines, drug resistance index in vitro through IC50 measurements and in vivo through the inhibition of tumor growth (TGI) which compare to those in the parental cell line. We successfully established Vemurafenib and Dabrafenib resistant A375 cell lines (A375 Vemurafenib R, A375 Dabrafenib R) with 21 and 31.5 folds of resistance index, respectively. The in vivo drug resistance efficacy was also tested by implanting Vemurafenib resistant cells subcutaneously into NOD-SCID mice and showed that A375 Vemurafenib R exhibited robust tolerance to 50 mg/kg Vemurafenib treatment (P.O.21 days, 50mpk, TGI=17.4%), whereas the parental cells were nearly completely inhibited (P.O.21 days, 50mpk, TGI=92.3%). In addition, single-cell sequencing of A375 Vemurafenib R revealed significant gene expression differences in the MAPK and EGFR signaling pathways, aiding in the identification of new targets, and signaling pathways associated with drug resistance, thereby providing potential targets for new drug development.
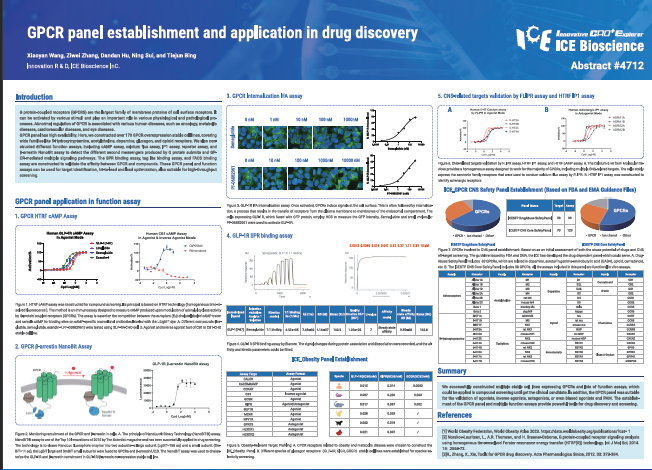
G protein-coupled receptors (GPCRS) are the largest family of membrane proteins of cell surface receptors. It can be activated by various stimuli and play an important role in various physiological and pathological processes. Abnormal regulation of GPCR is associated with various human diseases, such as oncology, metabolic diseases, cardiovascular diseases, and eye diseases. GPCR panel has high availability. Here, we constructed over 170 GPCR overexpression stable cell lines, covering wide families like 5-Hydroxytryptamine, acetylcholine, dopamine, glucagon, and opioid receptors. We also constructed different function assays, including cAMP assay, calcium flux assay, IP1 assay, reporter assay, and β-arrestin NanoBit assay to detect the different second messengers produced by G protein submits and GPCR-mediated multiple signaling pathways. The SPR binding assay, tag lite binding assay, and FACS binding assay are constructed to validate the affinity between GPCR and compounds. These GPCR panel and function assays can be used for target identification, hit-to-lead and lead optimization, also suitable for high-throughput screening.
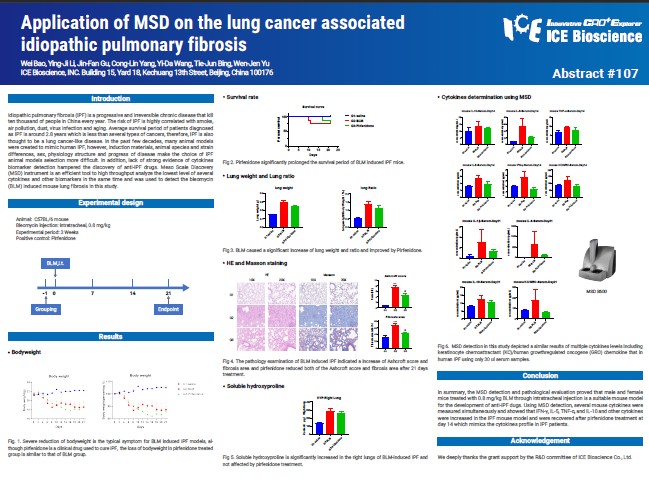
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible chronic disease that kill ten thousand of people in China every year. The risk of IPF is highly correlated with smoke, air pollution, dust, virus infection and aging. Average survival period of patients diagnosed as IPF is around 2.8 years which is less than several types of cancers, therefore, IPF is also thought to be a lung cancer-like disease. In the past few decades, many animal models were created to mimic human IPF, however, induction materials, animal species and strain differences, sex, physiology structure and progress of disease make the choice of IPF animal models selection more difficult. In addition, lack of strong evidence of cytokines biomarker detection hampered the discovery of anti-IPF drugs. Meso Scale Discovery (MSD) instrument is an efficient tool to high throughput analyze the lowest level of several cytokines and other biomarkers in the same time and was used to detect the bleomycin (BLM) induced mouse lung fibrosis in this study.