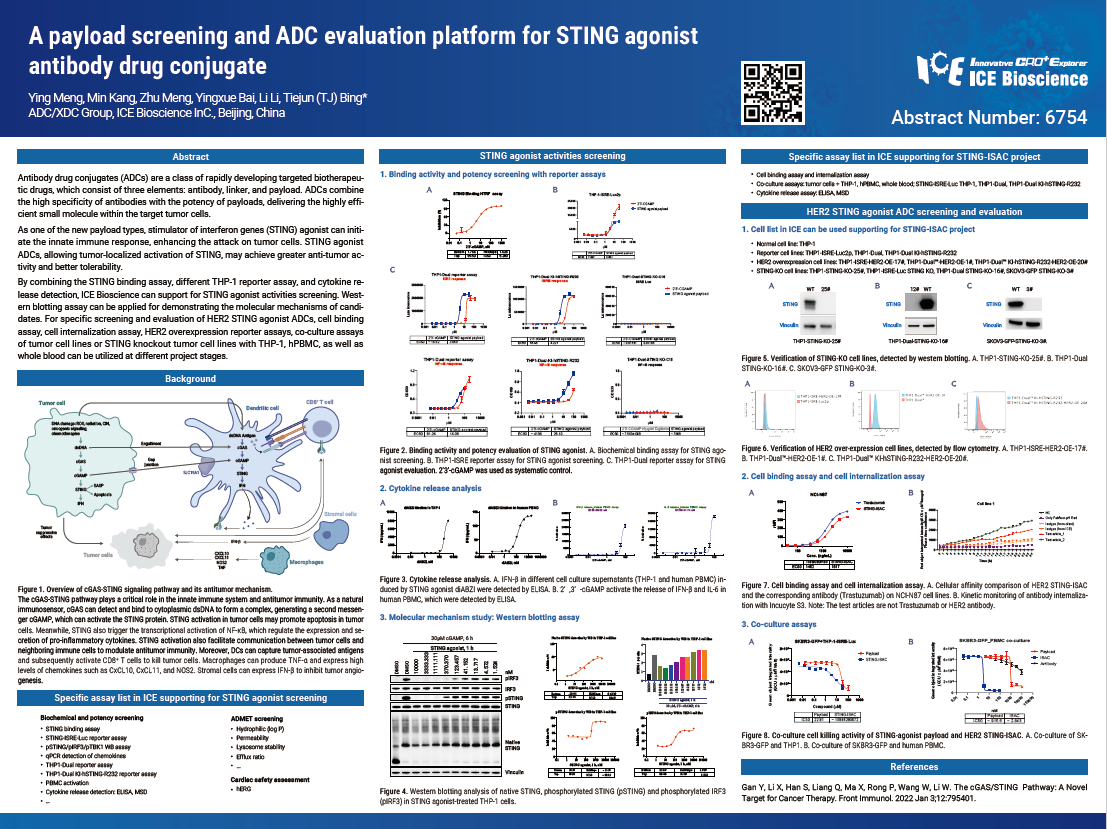
Antibody drug conjugates (ADCs) are a class of rapidly developing targeted biotherapeu- tic drugs, which consist of three elements: antibody, linker, and payload. ADCs combine the high specificity of antibodies with the potency of payloads, delivering the highly effi- cient small molecule within the target tumor cells.
As one of the new payload types, stimulator of interferon genes (STING) agonist can initi- ate the innate immune response, enhancing the attack on tumor cells. STING agonist ADCs, allowing tumor-localized activation of STING, may achieve greater anti-tumor ac- tivity and better tolerability.
By combining the STING binding assay, different THP-1 reporter assay, and cytokine re- lease detection, ICE Bioscience can support for STING agonist activities screening. West- ern blotting assay can be applied for demonstrating the molecular mechanisms of candi- dates. For specific screening and evaluation of HER2 STING agonist ADCs, cell binding assay, cell internalization assay, HER2 overexpression reporter assays, co-culture assays of tumor cell lines or STING knockout tumor cell lines with THP-1, hPBMC, as well as whole blood can be utilized at different project stages.

Molecular glue degraders (MGDs) induce the proximity of target proteins to E3 ubiquitin ligases, leading to target protein ubiquitination and subsequent degradation by the proteasome. Unlike conventional inhibitors that target enzymatic activity, these molecules enable the degradation of proteins previously deemed "undruggable," thereby rendering them "druggable". With a first-in-class VAV1-directed MGDs (MRT6160) enters in clinical phase I, VAV1 degraders showed the remarkable potential in Immunology and inflammatory diseases such as rheumatoid arthritis and colitis. Current advancements highlight its ability to reduce proinflammatory cytokine production, inhibit pathogenic T cell polarization, and mitigate autoimmune responses.
In the development of new drugs, it is crucial to know the safety profile of drug candidates in advance, as this can prevent the drug from being withdrawn from the market due to safety concerns after launch, thus reducing the risk for pharmaceutical companies.
It is estimated that approximately 75% of all adverse drug reactions (ADRs) are dose-dependent type A reactions, which can be predicted according to the pharmacological profiles of drug candidates. The pharmacological profiles are mainly divided into primary effects, which are related to the action of the compound at its intended target, and secondary effects, which arise from interactions with non-primary targets, i.e. off-target effects. Off-target interactions are often the cause of ADRs in animal models or clinical studies, so careful characterization and identification of the secondary pharmacological profiles of drug candidates early in the drug discovery process could help reduce the incidence of type A ADRs.
The major safety concerns that lead to the termination of clinical drug development programs and the withdrawal of approved drugs from the market are related to the cardiovascular system and the liver. The most frequent cardiovascular adverse event that prompts drug withdrawals is the potentially life-threatening cardiac arrhythmia known as Torsades de Pointes (TdP). Sunitinib, a multi-targeted receptor tyrosine kinase inhibitor, has shown promise in treating various tumors but raises concerns regarding its cardiovascular safety. This study aims to comprehensively evaluate the proarrhythmic risks associated with Sunitinib, employing an integrated platform that spans both in vitro and ex vivo analyses.