ICE Bioscience offers advanced safety panel screening services designed to identify potential off-target interactions and adverse drug reactions (ADRs) early in the drug development process. Our latest addition, the ICESTP™ Drug Abuse Safety Panel, is specifically tailored to assess the abuse potential of new drug candidates, addressing a critical aspect of drug safety.
Why Design the ICESTP™ Drug Abuse Safety Panel?
The need for the ICESTP™ Drug Abuse Safety Panel stems from the increasing recognition of drug abuse potential as a significant factor in the development and regulatory approval of new drugs. Drug products with abuse potential generally contain substances that affect the central nervous system (CNS) and can produce euphoria, hallucinations, or other mood-altering effects. These properties raise concerns about the risk of misuse, addiction, and public health implications.
The U.S. Food and Drug Administration (FDA) has provided guidelines emphasizing the importance of evaluating abuse potential early in the drug development process to prevent the introduction of products that could contribute to drug abuse epidemics. The FDA's "Assessment of Abuse Potential of Drugs" guidance outlines the necessity for thorough assessments of new drug candidates that are CNS-active, requiring a comprehensive battery of studies to determine their abuse liability.
Diverse Functional Screening: Traditional receptor-ligand binding studies are crucial, but functional assays provide additional insights into the drug's activity as either an agonist or antagonist at its target sites. This dual-mode functional assessment helps in understanding the precise pharmacological actions that could lead to abuse.
Advanced Functional Assays: Employs state-of-the-art functional assays to assess the pharmacological activity of drug candidates, across a wide range of CNS targets, including receptors, ion channels, and transporters, providing detailed profiles of potential abuse-related effects.
Alignment with FDA Guidelines: Designed in accordance with FDA's recommendations for assessing drug abuse potential, ensuring compliance with regulatory standards.
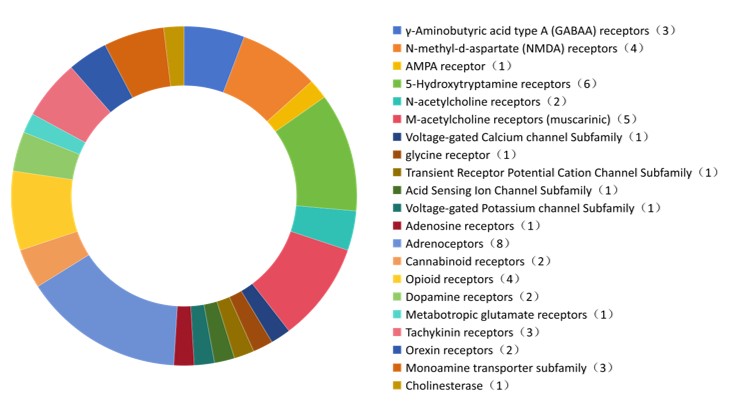
The ICESTP™ Drug Abuse Safety Panel features a comprehensive suite of functional assays to thoroughly evaluate the abuse potential of drug candidates. This panel includes 55 diverse CNS targets and conducts a total of 101 assays, providing detailed insights into both agonist and antagonist modes of action. The extensive coverage ensures a robust assessment of the pharmacological activity, helping to identify potential abuse liabilities early in the drug development process.
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.