In the realm of drug discovery and development, herg screening plays a pivotal role in ensuring the safety and efficacy of new pharmaceutical compounds. The HERG (Human Ether-à-go-go-Related Gene) channel is a critical component in cardiac physiology, and its inhibition can lead to severe cardiac side effects, including arrhythmias. Therefore, assessing potential drug interactions with the HERG channel is a crucial step in the drug development pipeline. By integrating HERG testing early in the drug discovery process, pharmaceutical companies can identify and mitigate potential cardiac risks, thereby increasing the likelihood of successful drug approval. This proactive approach not only enhances patient safety but also streamlines the overall drug development process.
Table of content:
The Impact of HERG Testing on Drug Approval Success Rates
HERG Screening: A Vital Component of Cardiovascular Risk Assessment
How HERG Test Results Influence Ion Channel Screening Decisions
Integrating HERG Testing into Comprehensive Drug Safety Protocols
HERG testing is a fundamental aspect of the drug approval process, as it directly influences the safety profile of new therapeutic agents. The ability to predict and prevent adverse cardiac events is essential for gaining regulatory approval from bodies such as the FDA. Drugs that exhibit HERG channel inhibition are at a higher risk of being rejected due to the potential for causing life-threatening arrhythmias. As a result, comprehensive HERG testing is not only a regulatory requirement but also a strategic advantage for pharmaceutical companies. By identifying compounds with unfavorable HERG profiles early on, developers can either modify the chemical structure or abandon the development of high-risk drugs, thus saving time and resources. Ultimately, robust HERG testing protocols contribute to higher success rates in drug approval by ensuring that only the safest compounds reach the market.
HERG screening is indispensable in evaluating the cardiovascular safety of new drugs. The HERG channel is responsible for the repolarization phase of the cardiac action potential, and its inhibition can lead to prolonged QT intervals, a marker for potential cardiac arrhythmias. By incorporating HERG screening into the early stages of drug development, researchers can effectively assess the cardiovascular risk associated with new compounds. This early assessment is crucial, as it allows for the identification of potentially hazardous drugs before they reach clinical trials. Moreover, HERG screening provides valuable data that can guide the optimization of drug candidates, ensuring that only those with the most favorable safety profiles proceed further in development. In this way, HERG screening serves as a critical tool in safeguarding patient health and improving the overall success rate of new drug approvals.
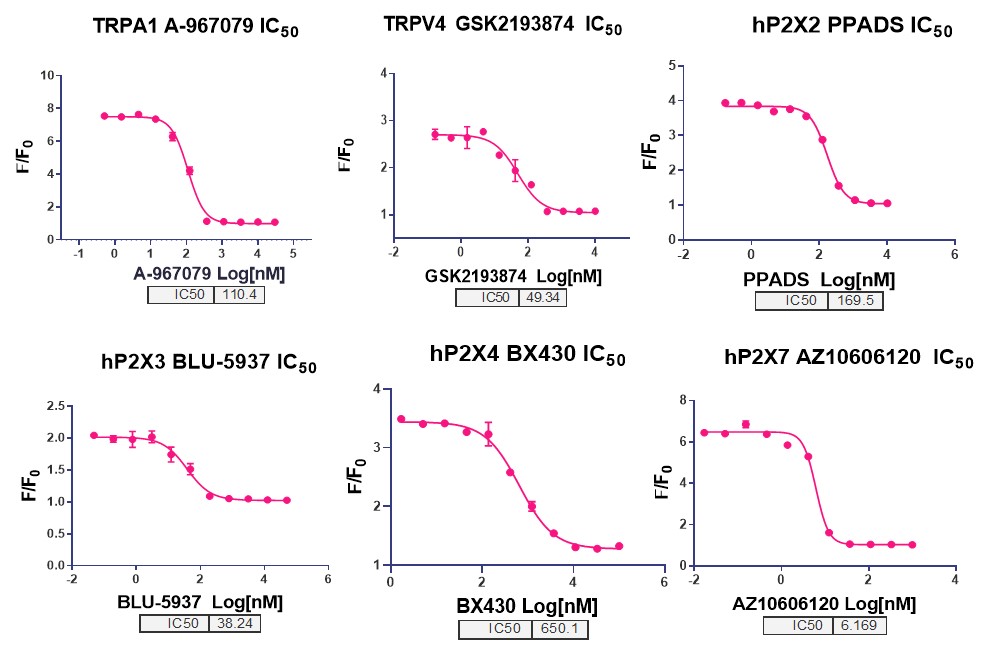
The results of a HERG test are instrumental in shaping the direction of ion channel screening efforts. By providing detailed insights into a compound's interaction with the HERG channel, these tests enable researchers to make informed decisions about which compounds to prioritize for further development. HERG test results can highlight potential safety concerns, prompting researchers to either modify the compound's structure or focus on alternative candidates with more favorable profiles. This data-driven approach ensures that resources are allocated efficiently, maximizing the chances of developing safe and effective drugs. Additionally, HERG test results can inform the design of subsequent studies, such as those involving other ion channels, by identifying key areas of concern that need to be addressed. Ultimately, the integration of HERG test data into the decision-making process enhances the overall efficiency and effectiveness of ion channel screening programs.
Incorporating HERG testing into comprehensive drug safety protocols is essential for ensuring the successful development of new pharmaceuticals. By systematically evaluating the potential cardiac risks associated with new compounds, HERG testing provides a critical layer of safety assessment that complements other screening efforts. This integration allows for a holistic approach to drug safety, wherein potential risks are identified and addressed at multiple stages of development. By doing so, pharmaceutical companies can reduce the likelihood of late-stage failures due to unforeseen cardiac issues, thus minimizing financial losses and ensuring that only the safest drugs reach the market. Furthermore, the inclusion of HERG testing in safety protocols demonstrates a commitment to patient safety and regulatory compliance, strengthening the overall integrity of the drug development process.
In summary, HERG screening is an indispensable component of modern drug development, offering critical insights into the cardiovascular safety of new compounds. By prioritizing HERG testing early in the development process, pharmaceutical companies can enhance their chances of regulatory approval while ensuring patient safety. The integration of HERG test results into ion channel screening decisions and comprehensive safety protocols further underscores the importance of this vital assessment tool. As the pharmaceutical industry continues to evolve, the role of HERG screening in safeguarding public health and advancing drug discovery will remain paramount.
2026-02-04
2026-02-04
2026-02-04
2026-02-04
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.