In the ever-evolving landscape of drug development, the ICESTP Safety Panel has emerged as a groundbreaking tool for enhancing preclinical safety assessments. This innovative approach to drug screening represents a significant leap forward in the field of pharmacology, offering researchers and pharmaceutical companies a more comprehensive and efficient method for identifying potential off-target effects of drug candidates. By leveraging cutting-edge technology and a deep understanding of cellular and biochemical responses, the ICESTP Safety Panel is revolutionizing the way we approach drug safety, ultimately leading to safer and more effective treatments for patients worldwide.
Table of contents:
Advancing Drug Safety with Cutting-Edge Safety Panel Screening Techniques
ICESTP 77: The First Fully Functional 77-Target Safety Panel on the Market
Streamlining the Drug Development Process with in vitro Safety Pharmacology Profiling
How ICESTP Safety Panels are Transforming Off-Target Drug Screening Services
The Safety Panels service provided by ICESTP represents a paradigm shift in preclinical safety assessment. By utilizing functional secondary pharmacology panels, these advanced screening techniques offer quantitative insights into potential off-target effects of drug candidates. The ICESTP Safety Panel, available in both 44 and 77 target configurations, is based on recent scientific consensus emphasizing the importance of early in vitro profiling. This approach allows researchers to identify off-target liabilities with unprecedented accuracy and efficiency. The Safety Panels service is designed to align with regulatory guidelines, ensuring that drug developers can meet the stringent safety requirements of governing bodies while streamlining their research processes.
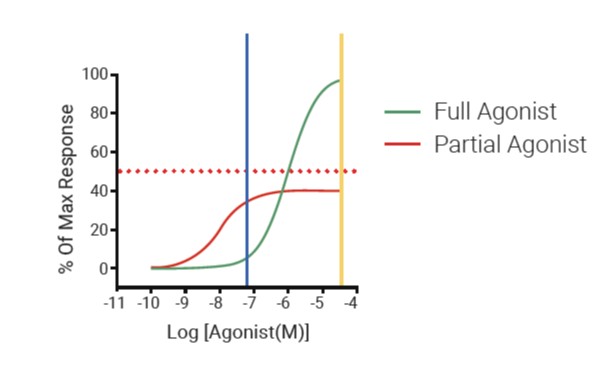
At the forefront of safety panel innovation is the ICESTP 77, the first fully functional 77-target safety panel available in the market. This comprehensive panel expands upon the core set of off-targets from the original Bowes-44 panel, incorporating additional relevant targets based on modern, data-driven selection methods. The ICESTP Safety Panel employs functional assays that measure actual cellular and biochemical responses, providing more predictive value than traditional binding assays. This approach allows researchers to gain a more nuanced understanding of potential drug interactions, ultimately leading to more informed decision-making throughout the drug development process. The Safety Panels service offers both single-concentration and full dose-response formats, with the latter providing quantitative IC50/EC50 values for a deeper analysis of drug behavior.
The ICESTP Safety Panel is designed to support decision-making across various preclinical stages, from early screening to pre-IND safety evaluation. By offering comprehensive, functional screening of potential off-target effects, the Safety Panels service helps reduce risks in drug development and accelerates the path to clinical trials. One of the key features of the ICESTP Safety Panel is its use of 1 mM ATP for kinase target profiling, which reflects physiological conditions and reduces false positives. This attention to detail ensures that researchers receive the most accurate and relevant data possible, enabling them to make informed decisions about which drug candidates to pursue further.
The impact of the ICESTP Safety Panel on off-target drug screening services cannot be overstated. By offering dual replicates at top dose, dual data visualizations, and expert interpretation in visual reports, the Safety Panels service provides researchers with a wealth of valuable information. The availability of two panel options – ICESTP 44 and ICESTP 77 – allows researchers to choose the level of detail that best suits their needs and stage of development. This flexibility, combined with the comprehensive nature of the panels, is transforming the way pharmaceutical companies approach preclinical safety assessments. The ICESTP Safety Panel is not just a tool for identifying potential risks; it's a catalyst for innovation in drug development, enabling researchers to explore new chemical spaces with greater confidence and efficiency.
In conclusion, the ICESTP Safety Panel represents a significant advancement in preclinical safety assessment, offering a comprehensive and functional approach to identifying potential off-target effects of drug candidates. The Safety Panels service provided by ICESTP is revolutionizing the drug development process, enabling researchers to make more informed decisions and reduce risks associated with new treatments. By leveraging cutting-edge technology and a deep understanding of cellular and biochemical responses, the ICESTP Safety Panel is paving the way for safer, more effective drugs to reach patients faster. As the pharmaceutical industry continues to evolve, the role of innovative safety screening tools like the ICESTP Safety Panel will only become more crucial in ensuring the development of life-changing therapies while maintaining the highest standards of safety and efficacy.
2026-02-04
2026-02-04
2026-02-04
2026-02-04
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.