In our previous App Note, Comprehensive Profiling of STAT6 Degraders: From Binding and Ternary Complex Formation to Cellular Degradation and Pathway Suppression, we outlined a systematic in vitro framework to characterize STAT6 degraders across key stages and downstream pathway modulation. These studies addressed how effectively STAT6 degraders act on their intended target at the molecular and cellular levels.
Building on this foundation, deeper characterization becomes essential as programs advance toward lead optimization and translational decision-making. In particular, understanding selectivity across related targets, species-specific degradation behavior, and potential off-target effects is critical for evaluating both efficacy and developability.
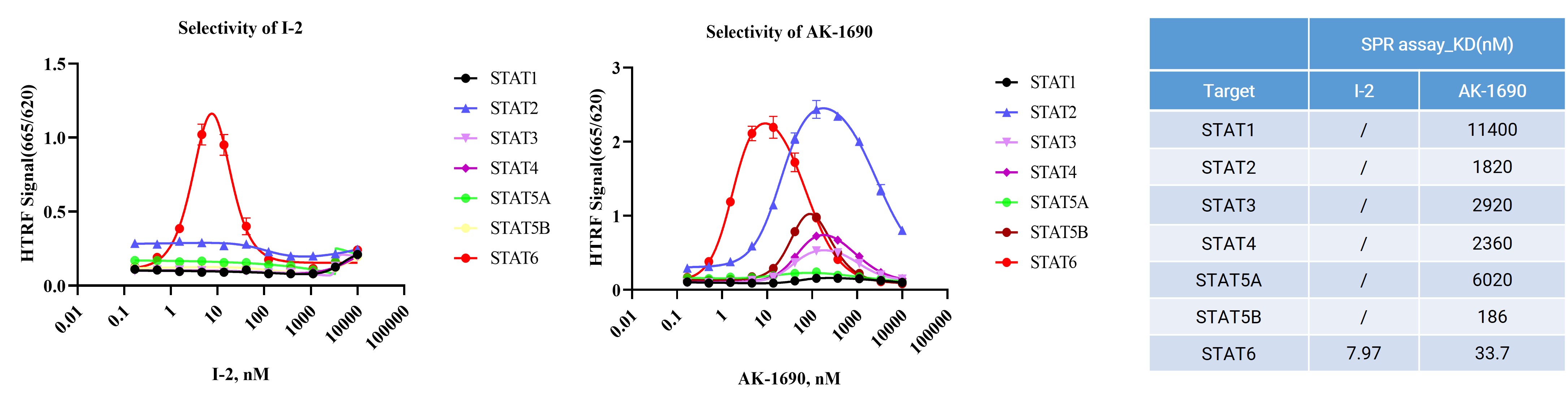
To directly assess selectivity at the level of complex formation, we employed TR-FRET and SPR–based assay to quantitatively measure the assembly of STAT family proteins, degraders, and the E3 ligase. By profiling multiple STAT family members side by side, this approach enables direct comparison of degrader-induced ternary complex formation and POI-degrader binding, providing a mechanistic readout of selectivity prior to downstream studies.
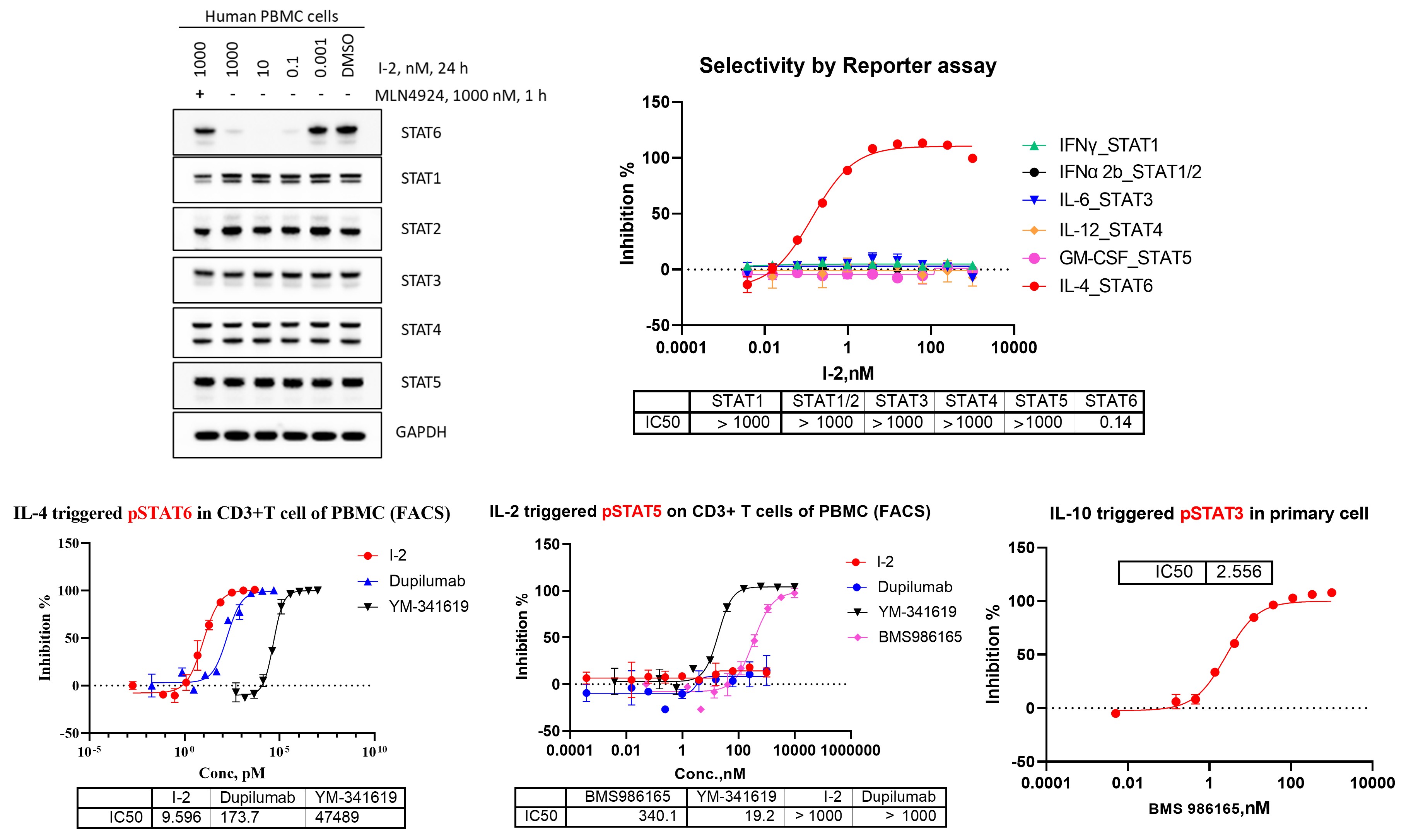
To complement binding–based selectivity assessment, downstream validation at the protein and signaling levels is essential for confirming functional specificity within the STAT family. We employed Western blot analysis to directly evaluate degrader-induced modulation of reporter gene levels, alongside phosphorylation-based functional assays to assess pathway engagement. By integrating reporter intensities and pSTAT measurements across multiple STAT members, including cellular and immune-relevant contexts, this approach provides orthogonal evidence of STAT6 selectivity and helps distinguish true on-target activity from broader STAT pathway perturbation.
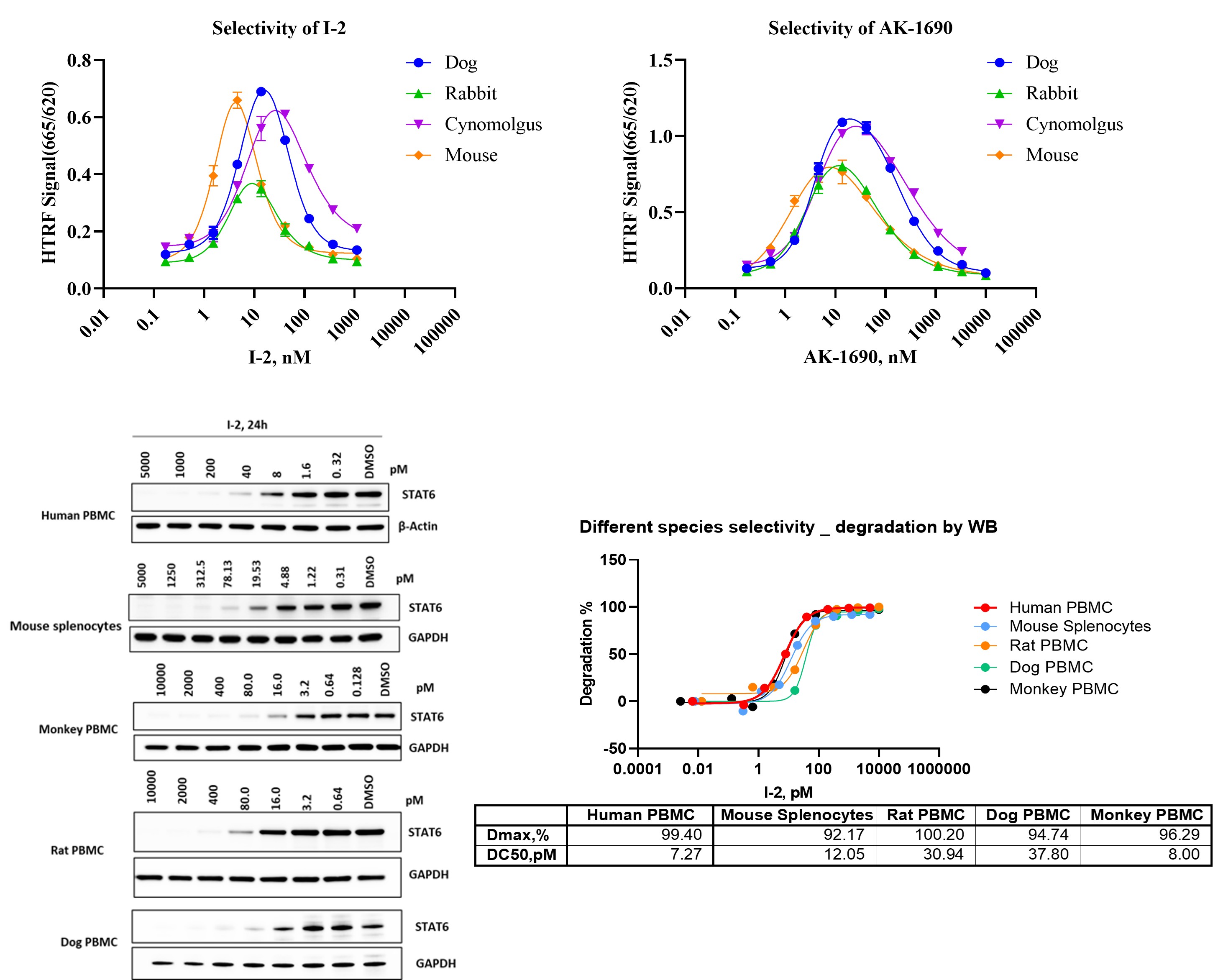
Species-specific behavior is a critical consideration in the development of STAT6 degraders, as differences in target sequence, E3 ligase compatibility, and cellular context can significantly influence degradation outcomes. Here, we present a cross-species in vitro profiling strategy integrating TR-FRET ternary complex formation, SPR-based binary binding, and Western blot–based degradation analysis across human, mouse, rat, rabbit, dog, and monkey systems. By correlating biochemical engagement with cellular degradation potency in primary immune cells, this approach enables systematic assessment of species-dependent activity and supports informed selection of translational and preclinical models for STAT6 degrader programs.
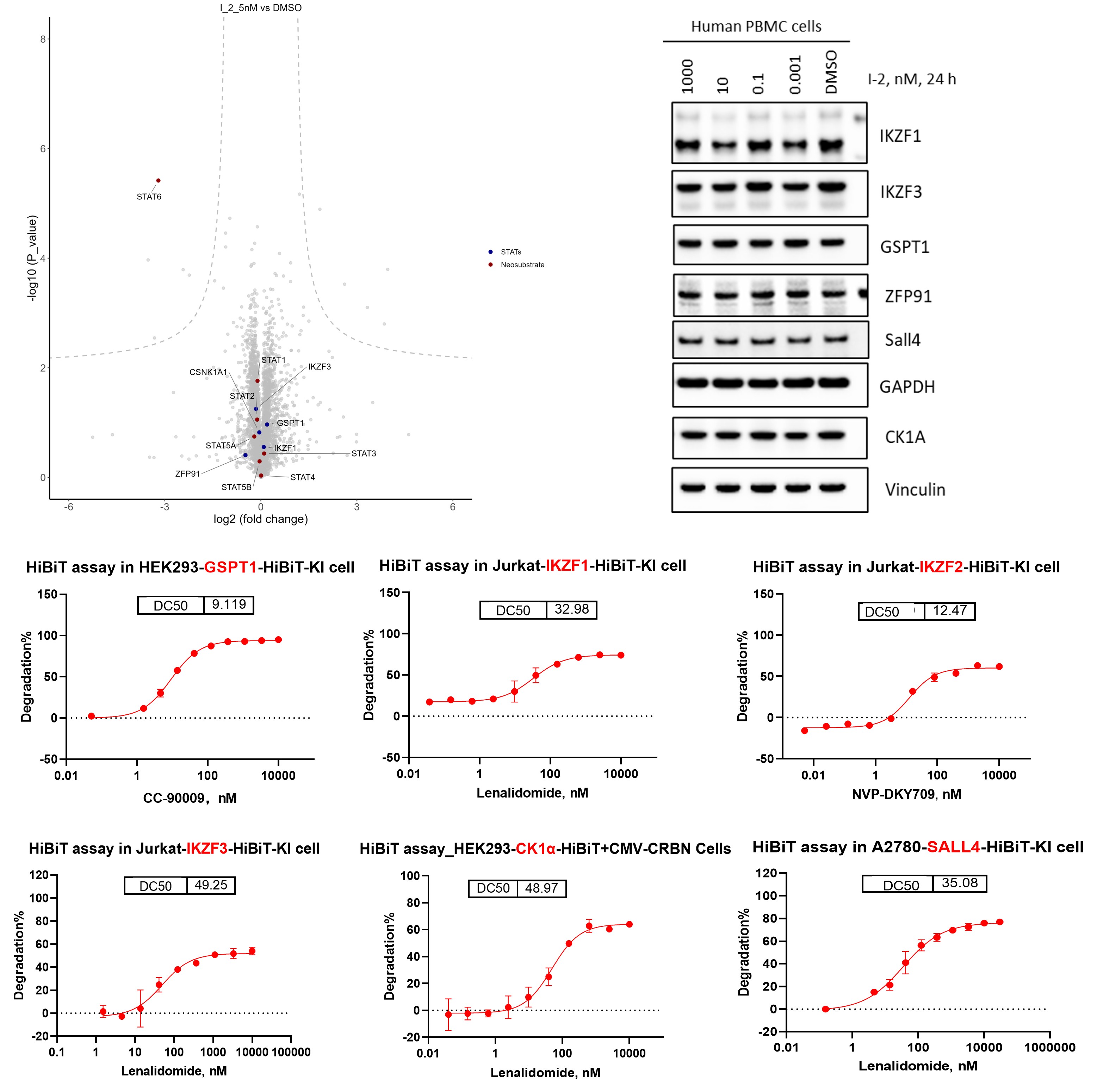
Off-target substrate degradation remains a key risk in CRBN-based degrader programs and requires systematic evaluation beyond the intended target. Here, we applied a multi-layered in vitro profiling strategy combining Western blot analysis, DIA-based proteomics, and HiBiT reporter assays to identify and validate potential CRBN off-target substrates. By integrating unbiased proteomic screening with targeted, quantitative cellular readouts, this approach enables efficient detection, confirmation, and prioritization of off-target degradation events, supporting more informed optimization and risk assessment for CRBN-recruiting degraders.
Early evaluation of safety liabilities is essential for advancing small-molecule and degrader programs with confidence. To assess potential off-target pharmacology and cardiac risk, we applied the ICESTP SafetyPanel™ 77 alongside a dedicated hERG patch clamp assay to profile compound activity across a broad range of clinically relevant targets. The safety panel provides a comprehensive view of receptor, ion channel, and enzyme engagement at a fixed concentration, while the hERG assay enables focused evaluation of cardiac ion channel inhibition across a concentration range.
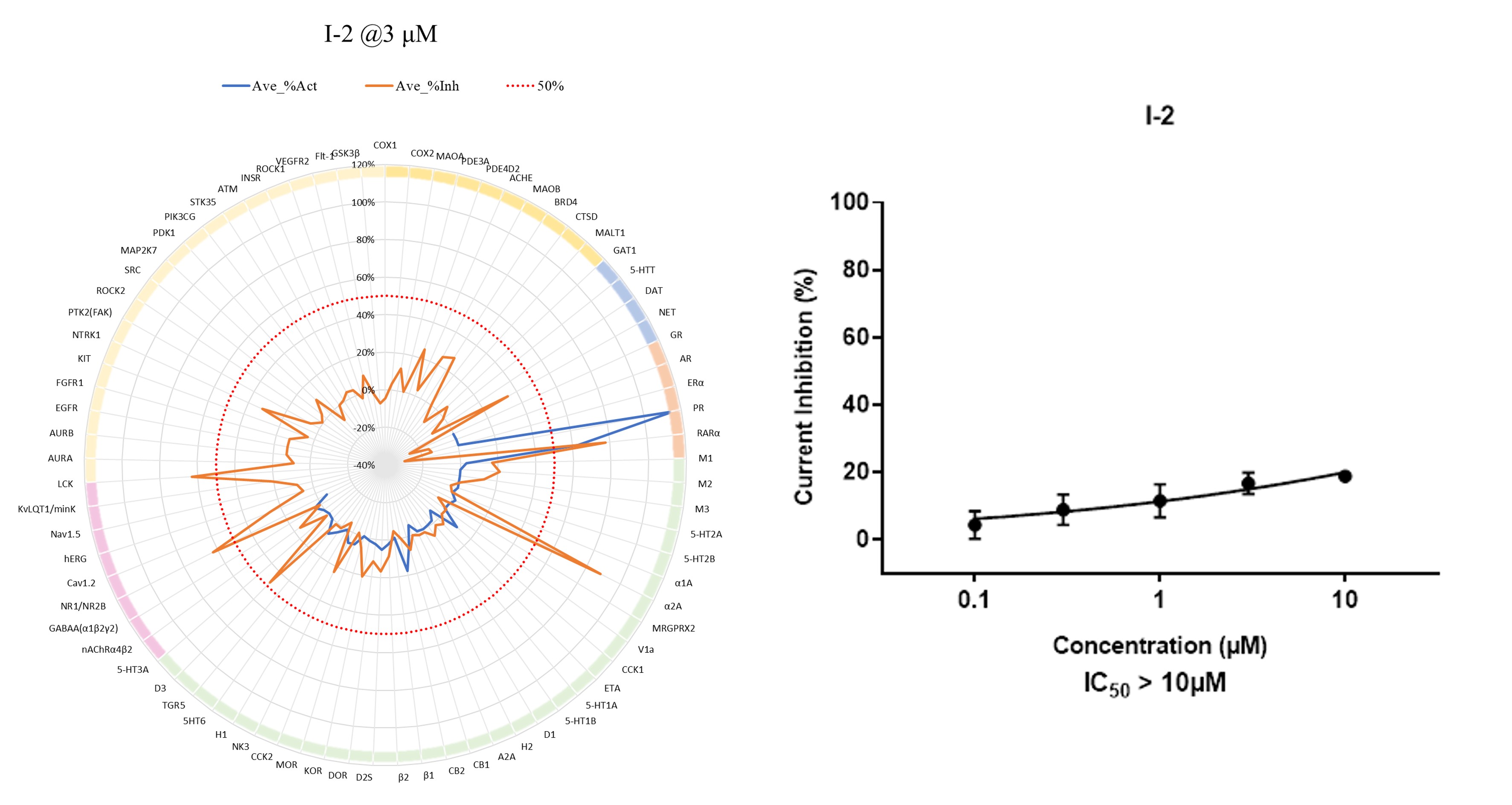
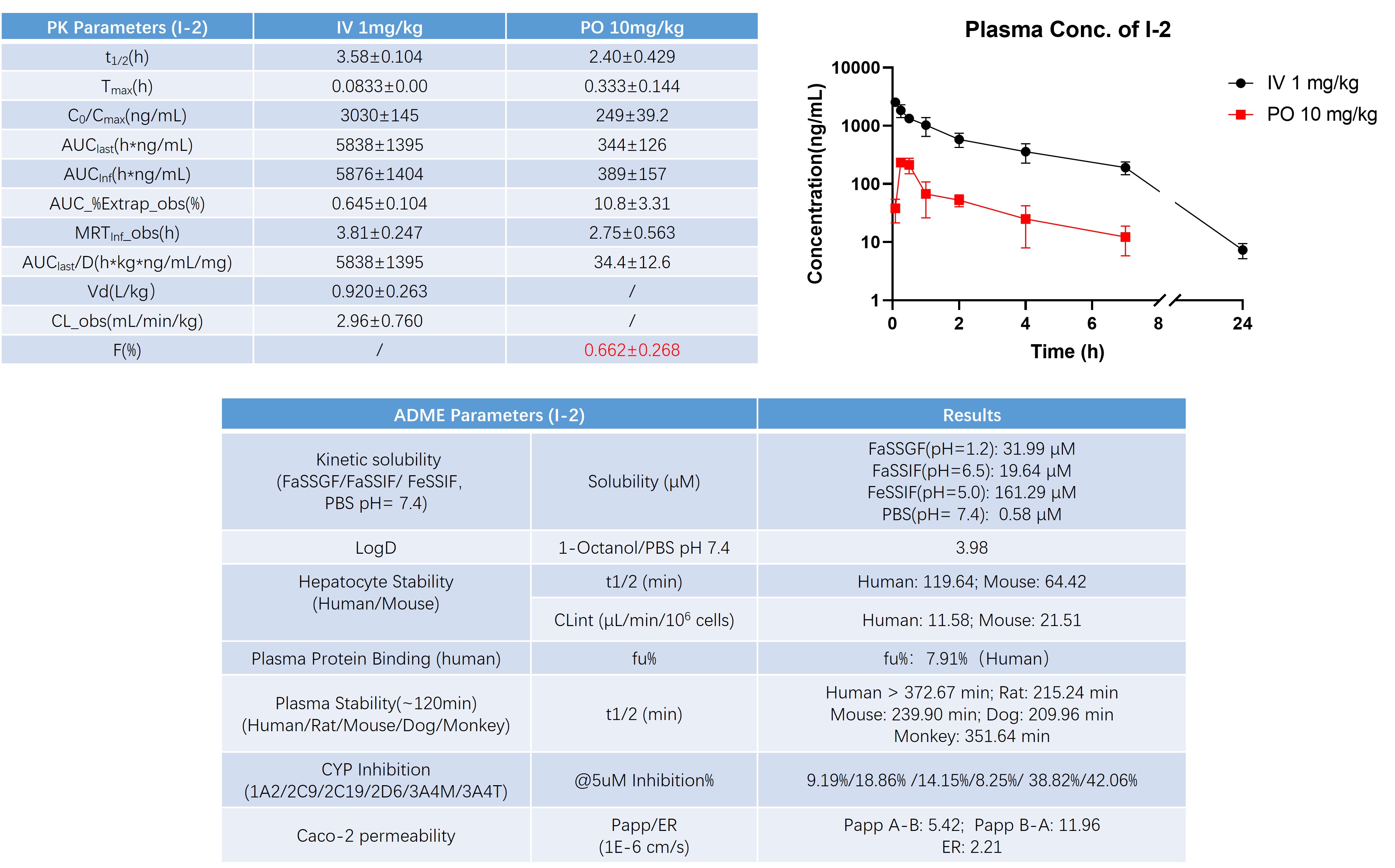
To support translational assessment and dose selection, we conducted a comprehensive evaluation of the pharmacokinetic and ADME properties of I-2. In vivo PK studies following intravenous and oral administration were performed to characterize systemic exposure, clearance, distribution, and oral bioavailability, while in vitro ADME profiling assessed solubility, metabolic stability, plasma protein binding, CYP inhibition, and permeability across relevant systems.
Together, these studies establish a comprehensive in vitro and in vivo profiling framework for STAT6 degrader programs. This approach enables systematic evaluation of both efficacy and developability risks. Such multidimensional profiling supports informed optimization, model selection, and translational decision-making, providing a robust foundation for advancing STAT6 degraders toward preclinical development.
2025-10-30
2025-09-28
2025-08-19
2025-08-15
We value your inquiries and are here to provide you with tailored solutions for your drug discovery and development needs. Whether you have questions, require more information, or are interested in discussing potential collaborations, our team of experts is just a message away.
Feel free to reach out to us.